Will this antibiotic work? ASU scientists develop rapid bacterial tests

Shaopeng Wang, an associate professor of biomedical engineering in the School of Biological and Health Systems Engineering, part of the Ira A. Fulton Schools of Engineering at Arizona State University, pictured in his lab. Wang has earned a patent for his rapid antimicrobial susceptibility testing that uses video-based object scattering intensity detection. Courtesy photo
Bacteria multiply at an astonishing rate, sometimes doubling in number in under four minutes. Imagine a doctor faced with a patient showing severe signs of infection. As they sift through test results, a question looms: Will this antibiotic work?
Ten years ago, Shaopeng Wang — an associate professor of biomedical engineering in the School of Biological and Health Systems Engineering, part of the Ira A. Fulton Schools of Engineering at Arizona State University, jointly appointed with the ASU Biodesign Center for Bioelectronics and Biosensors and a researcher at Mayo Clinic — tackled a critical need in diagnostic medicine: the rapid identification of bacterial infections and the determination of antibiotic susceptibility.
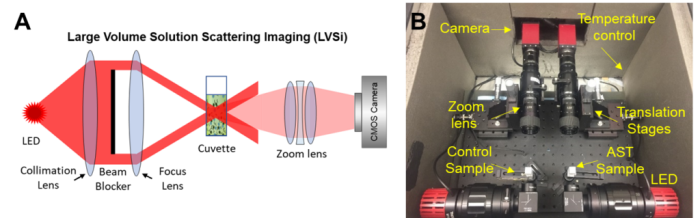
Wang recently earned a patent for his innovative method that uses video-based object scattering intensity detection, a technique that helps doctors see bacterial activity, to rapidly test clinical urine samples. The method offers a faster and more precise approach to combating infections than traditional testing. Wang’s goal is to implement the technology in doctor’s offices around the world to improve patient care.
Determining an effective course of treatment
Antibiotics work by targeting and damaging essential parts of bacteria, such as a cell wall or DNA, and destroying the undesirable organism.
However, resistant strains can survive and continue to reproduce even while other bacteria are eliminated. Repeated use of the same antibiotics intensifies the selection of these resistant strains, allowing them to thrive.
Antibiotic susceptibility tests are essential tools in clinical laboratories for determining whether specific antibiotics effectively treat infections in the human body. They are critical in guiding clinical treatment decisions.
However, culturing for bacterial strains — the current gold standard for diagnosing bacterial infections, such as those responsible for fevers — can take up to three days.
During this waiting period, patients suffer from uncertainty while physicians determine a broad-spectrum antibiotic to use to destroy the bacteria causing infection.
Physicians often prescribe broad-spectrum antibiotics that could be effective while they wait for results that confirm the type of bacteria and its drug resistance. The waiting game risks ineffective treatment and exacerbates the ongoing crisis of antibiotic resistance.
Antibiotic resistance can occur when bacteria survive treatment and multiply their resistant properties. In other cases, antibiotics may not be the best course of action, such as when treating a patient with a viral infection.
To address these issues, Wang wanted to eliminate the current testing model that consists of taking a culture step, typically extending the diagnostic timeline for up to three days. Instead, his team of researchers has developed an innovative method that directly analyzes urine samples.
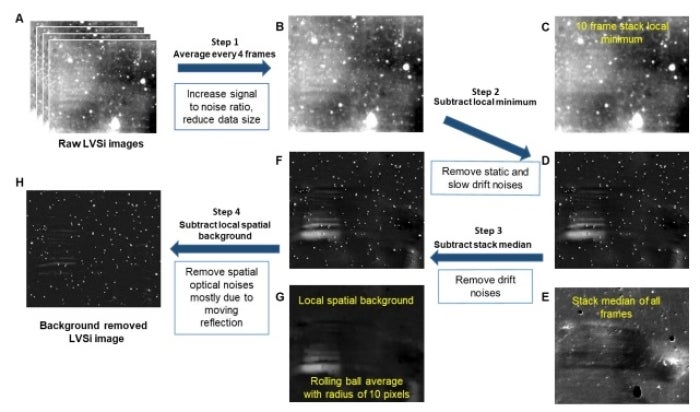
“We use a scattering image, similar to looking at stars in the night, to measure the intensity of scattered light,” Wang says. “As bacteria grow, the scattered light becomes brighter, allowing us to track changes in bacterial size and numbers without needing any labels.”
Understanding antibiotic effectiveness in 90 minutes
Urinary tract infections, or UTIs, are a major health care burden, according to a study published in the American Journal of Infection Control. These bacterial infections are more common in women than in men, with approximately 50% to 60% of women being affected across their lifespan.
Shelley Haydel is a professor of microbiology in the ASU School of Life Sciences and a researcher at the ASU Biodesign Center for Bioelectronics and Biosensors. Over the course of a decade, her work has focused on improving how we detect bacterial infections and determine their susceptibility to antibiotics in a much shorter time.
“E. coli is the most common cause of uncomplicated urinary tract infections, but there are about eight microorganisms responsible for most cases,” Haydel says. “We identified infections within 30 minutes and determined antibiotic susceptibility in 90 minutes, with 98% accuracy compared to the standard three-day process.”
The new method begins by filtering the urine sample to remove large particles and mixing it with nutrients and varying doses of antibiotics. Using a low-magnification 2x microscope, researchers can observe bacterial growth or its absence.
Within 90 minutes, E. coli bacteria cells increase rapidly, indicating that the antibiotic is ineffective, or they do not grow, suggesting that the antibiotic is successfully inhibiting the bacteria. This approach differs from traditional diagnostic methods, which rely on optical density measurements and require large bacterial populations that need culture-based sample enrichment.
Postdoctoral researcher Jiapei Jiang, who works in Wang’s lab, is focused on advancing diagnostic tools for bacterial infections, with a particular emphasis on sepsis and urinary tract infections.
“Bacteria-causing sepsis are often the same as those causing UTIs, but precise diagnosis still takes about a week. During that time, because sepsis is life-threatening, doctors have no choice but to flood your blood with high concentrations of antibiotics,” Jiang says. “Even if you survive, your internal microbiome system is disrupted and takes a long time to recover.”
Wang’s process allows for detection at the individual bacterial level. For instance, E. coli can double every 20 minutes, enabling the team to detect bacterial growth or inhibition in a significantly shorter time frame.
Using a low-zoom microscope enables the team to observe a larger sample volume, ensuring that even low concentrations of bacteria can be detected. Their innovative scattering imaging technique tracks bacterial growth by measuring changes in light intensity, eliminating the need for labels or dyes that could complicate the analysis.
Impact and future outlook
This pioneering approach aims to provide clinicians with accurate diagnostic information on the first day of testing, empowering them to prescribe the correct antibiotic immediately.
The potential benefits extend beyond more effective treatments; this method could also mitigate the misuse of antibiotics, a significant driver of the rise in drug-resistant bacteria.
Wang and his team aspire to make this technology accessible and widely adopted within the health care landscape, ensuring that bacterial infections are treated more effectively while simultaneously addressing the long-term threat posed by antibiotic resistance.
As the researchers continue their vital work, the promise of faster, more accurate diagnostics could fundamentally transform how bacterial infections are managed, heralding a new era in medical care.
More Science and technology

ASU-led space telescope is ready to fly
The Star Planet Activity Research CubeSat, or SPARCS, a small space telescope that will monitor the flares and sunspot activity of low-mass stars, has now passed its pre-shipment review by NASA.…

ASU at the heart of the state's revitalized microelectronics industry
A stronger local economy, more reliable technology, and a future where our computers and devices do the impossible: that’s the transformation ASU is driving through its microelectronics research…

Breakthrough copper alloy achieves unprecedented high-temperature performance
A team of researchers from Arizona State University, the U.S. Army Research Laboratory, Lehigh University and Louisiana State University has developed a groundbreaking high-temperature copper alloy…