X-ray light reveals how virus responsible for COVID-19 covers its tracks, eluding the immune system

Image of NendoU, a protein used by the SARS CoV-2 virus to hide from the immune system. NendoU performs this feat by binding with viral RNA in the infected cell and cutting off a terminal portion of the sequence that the immune system uses to identify the presence of a foreign invader. Graphic by Jason Drees
The COVID-19 pandemic, caused by the SARS CoV-2 virus that killed over 1 million Americans since 2020, continues to threaten populations around the world. In recent weeks, XBB.1.5, the most transmissible variant to date, has started to sweep across the country.
One aspect of the novel coronavirus that makes it so infectious and challenging to control is its ability to outwit the body’s innate immune defenses.
A new study examines NendoU (pronounced nenn-doh-YOU), a viral protein responsible for the virus’s tactics of immune evasion. The structure of this crucial protein is explored in detail, using a technique known as serial femtosecond X-ray crystallography.
For the first time, the NendoU protein is imaged to a high resolution of 2.5 angstroms at room temperature. The resulting structure reveals underlying details of the protein’s flexibility, dynamics and other features with unprecedented clarity. Such structural information is crucial in the design of new drugs and may help advance therapeutics to target SARS CoV-2.
“Our study focuses on how COVID-19 hides from the immune system using the NendoU protein,” says Rebecca Jernigan, first author of the study and a researcher with the Biodesign Center for Applied Structural Discovery at Arizona State University. “As we better understand the structure and mechanics of NendoU, we have a better idea of how we can design antiviral drugs against it.”
The Biodesign Center for Applied Structural Discovery has made critical advances in structural studies of this kind, solving a variety of complex biological structures. The center is directed by Petra Fromme, who is a Regents Professor with ASU’s School of Molecular Sciences and the lead investigator of the study, which was funded by a RAPID grant from the National Science Foundation and the BioXFEL Science and Technology Center.
“This work is so exciting as it shows for the first time that the differences in flexibility of the protein play an important role in the functional mechanism," Fromme says. "This will be critical for development of drugs against NendoU, with potential to reveal the presence of the virus to the immune system, which can then react and hinder serious infections.”
The new research appears in the current issue of the Cell Press journal Structure.
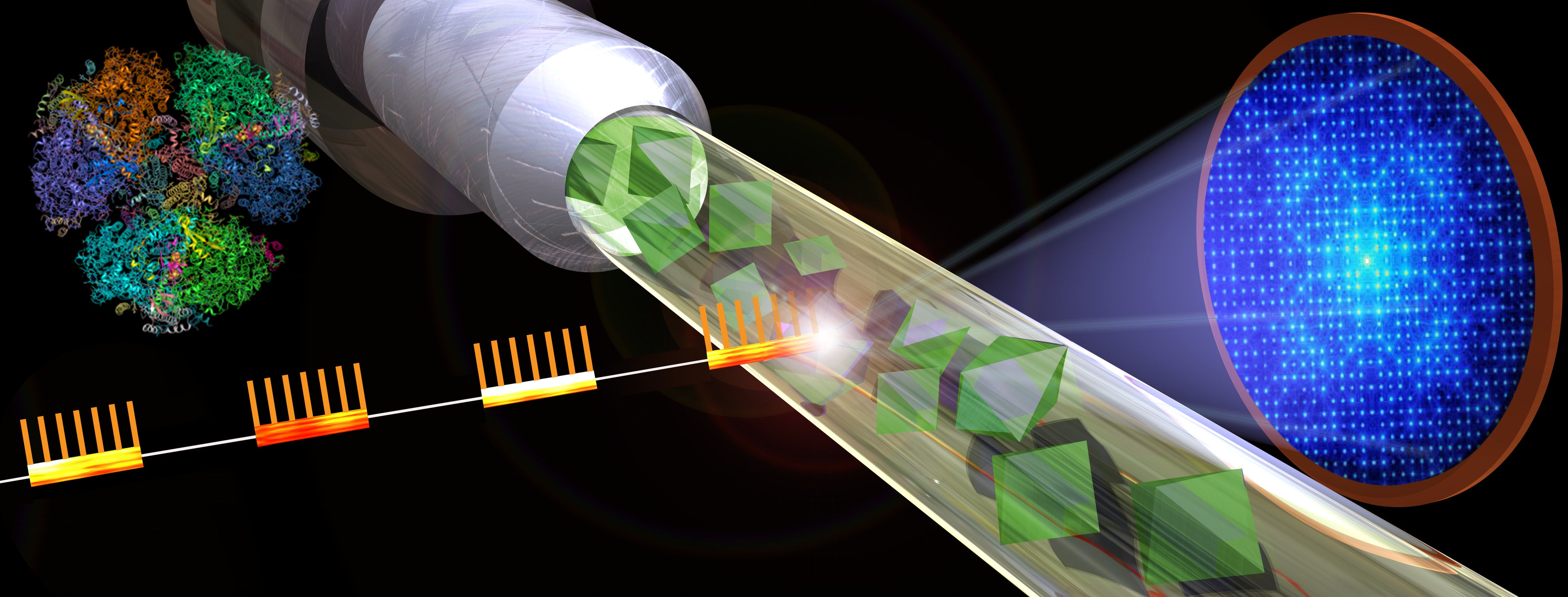
Graphic shows the basic design of a serial femtosecond crystallography experiment. X-ray bursts strike a jet of crystallized samples resulting in diffraction patterns that can be reassembled into detailed images. Graphic by Michael Northrop
Viral intrigue
Viruses have evolved complex strategies to elude the body’s defense mechanisms. Research points to a number of tactics used by the most virulent coronaviruses, a group of pathogens that includes those causing COVID-19 (SARS CoV-2), Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).

Rebecca Jernigan
The new study explores how the protein NendoU helps SARS CoV-2 hide from the immune system in plain sight. Once a virus binds to a receptor on the cell surface, it inserts its genetic material into the cell, causing the cell to manufacture multiple copies of the viral genome, consisting of either DNA or, in the case of coronaviruses, RNA.
When viruses like SARS CoV-2 replicate within cells, their growing RNA sequence produces a tail at the end, known as a poly-U tail. This tail is unique to RNA viruses.
Human cells are equipped with sensors that are fine-tuned to detect invading RNA viruses because the poly-U tail gives away their identity as foreign invaders, allowing the immune system to target them. Research has shown that SARS CoV-2 uses its NendoU protein to bind with, then cut off the poly-U tail. When the NendoU chews up the poly-U tail, this causes the virus to be less visible to the immune system.
Master of disguise
To thwart NendoU’s ability to conceal the virus, researchers need high-resolution images of the three-dimensional structure of the protein. Until now, solved structures of the NendoU protein have been completed only under cryogenic conditions, using a technique known as cryo-EM, in which the sample under study is flash-frozen and imaged with electron microscopy or by X-ray crystallography of large frozen crystals. This provided important clues about the precise nature of NendoU, but more information will be needed before a drug can be designed to inhibit NendoU and expose the SARS CoV-2 virus to immune targeting.
To accomplish this, researchers need to resolve the structure in such detail that they know where every atom in the protein is situated, and ideally the structure would be determined at conditions close to natural conditions at room temperature, where dynamics can be detected. However, damage by electrons or X-rays are severe, so that data collection is in most cases done under cryogenic conditions, where all movements are frozen. To obtain such atomic-scale resolution at room temperature, a specialized X-ray facility, known as an XFEL (for X-ray free electron laser), was required.

Petra Fromme
In the current study, researchers obtained the first snapshots on the path to an atomic-scale structure. The technique, known as serial femtosecond crystallography, involves crystallizing the protein sample in the form of billions of small microcrystals, then delivering them at room temperature in a jet to extremely short bursts of powerful X-ray light, producing a series of tens of thousands of diffraction patterns, each from a small microcrystal.
The ultrashort X-ray pulses, lasting just tens of femtoseconds, outrun the X-ray damage to the crystals, allowing for data to be collected at room temperature under near-physiological conditions. To give a sense of the extremely compacted time scale of these X-ray bursts, a femtosecond is equal to one quadrillionth of a second. Computers are used to combine large batches of these X-ray snapshots, allowing researchers to construct detailed, 3D structures of a protein and examine its dynamical behavior.
The current study was conducted using LCLS (Linac Coherent Light Source), the only X-ray free-electron laser in the U.S., at the SLAC National Accelerator Laboratory using the Macromolecular Femtosecond Crystallography instrument. The researchers used femtosecond X-ray crystallography to unlock the structure of the NendoU protein as it latched on to its substrate. In living cells, this would be the poly-U tail of the RNA strand, but for the study, a smaller molecule known as citrate was found in the RNA binding site.
"It was exciting to be invited to do an experiment at LCLS,” says Sabine Botha, co-corresponding author of the study and the project lead for data analysis. “They had just had a long shutdown period and reopened in the middle of the pandemic with a call for SARS-CoV-2 proposals. It was a very challenging experiment, with a brand-new X-ray detector, but also very rewarding.
Bringing NendoU into focus
One of the advantages of structural studies with XFELs is that biological phenomena can be studied close to their natural physiological state. The current findings reveal that the room temperature structure of the NendoU protein is more flexible compared to the cryogenic structure. This is likely a more faithful representation compared with the “frozen” structures earlier identified.

Sabine Botha
“Like the previous structures, we also saw that NendoU forms a hexamer (six identical NendoU proteins bound together),” says Debra Hansen, co-author of the paper and associate research professor with the Biodesign Center for Applied Structural Discovery. Additionally, the researchers found that one half of the protein had more flexibility than the other half, which was more rigid.
The structural details unveiled by the XFEL light show that NendoU acts through a two-step process. First, the more rigid half of the protein binds to the active site of the substrate (in this case, the citrate molecule). The flexible half of the hexamer also binds citrate (or the RNA) but less tightly. Once the rigid half performs the task of cleaving the RNA strand, it releases the strand. This rigid half then becomes flexible while the flexible half switches to a rigid state and the cycle is repeated. This scissor-like movement of NendoU’s two primary components helps to erase the telltale signal of the virus’ presence within the cell, disabling the immune response.
The XFEL snapshots of these movements provide a detailed map for eventual drug design. Future structures using room-temperature conditions will map these various movements, and each map will allow the most accurate computational design of COVID-fighting drugs.
The project involved the resources and talents of many research groups and more than 30 collaborators. In addition to the Biodesign Center for Applied Structural Discovery, ASU’s School of Molecular Sciences, Department of Physics and Fulton School of Electrical, Computer and Energy Engineering, contributors include the University of Buffalo; University of Wisconsin-Milwaukee; the Deutsches Elektronen-Synchrotron, Hamburg; Spanish National Research Council, Madrid; and Lawrence Livermore National Laboratory. A full list of author contributions can be found here.
More Science and technology

ASU professor honored with prestigious award for being a cybersecurity trailblazer
At first, he thought it was a drill.On Sept. 11, 2001, Gail-Joon Ahn sat in a conference room in Fort Meade, Maryland.…

Training stellar students to secure semiconductors
In the wetlands of King’s Bay, Georgia, the sail of a nuclear-powered Trident II Submarine laden with sophisticated computer…

ASU startup Crystal Sonic wins Natcast pitch competition
Crystal Sonic, an Arizona State University startup, won first place and $25,000 at the 2024 Natcast Startup Pitch Competition at…