ASU teams up with Phoenix Children’s and Valleywise Health to study vaccine effectiveness vs. influenza, COVID-19
Modern vaccines against infectious disease have saved hundreds of millions of lives around the world. Although they are the subject of enormous research, many puzzles remain.
Why do vaccines prevent illness in one individual while failing to provide the same level of protection in another? Which features of an individual’s medical history, immunological makeup, geographical location, age, gender and socioeconomic status contribute to vaccine effectiveness? What causes vaccine aversion in certain communities? How does post-vaccination protection evolve over time?
These are among the vital questions to be addressed in an ambitious, five-year, $12.5 million project undertaken by Arizona State University’s Biodesign Institute, in close collaboration with Phoenix Children’s, Valleywise Health and ASU Health Services.
The project builds on the ongoing, year-to-year efforts by the Centers for Disease Control and Prevention to evaluate how well influenza vaccines perform across populations and under a range of conditions. The new study will also include the evaluation of vaccine effectiveness against the SARS CoV-2 virus.
“Phoenix is a very fast-growing area with a diverse population, which is changing economically and demographically every day,” says Vel Murugan, principal investigator on the new CDC project and associate director of research and associate research professor with the Biodesign Institute at ASU. “So, this is probably the perfect time and location to do the kinds of studies we are going to do.”
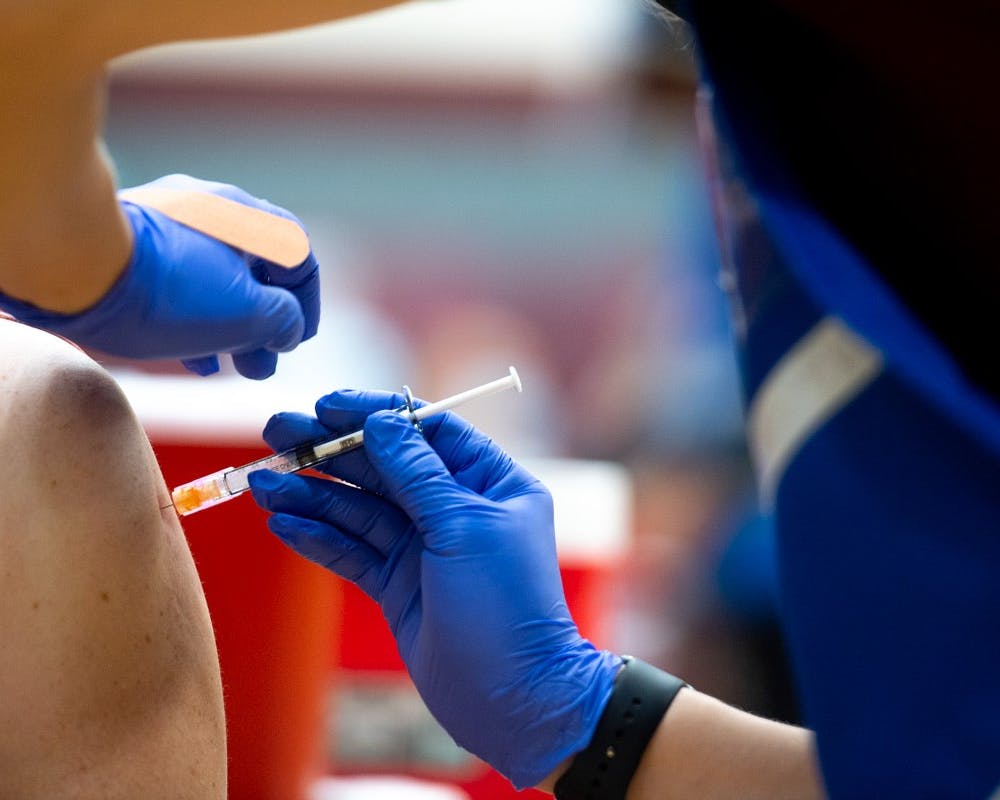
A volunteer administers the COVID-19 vaccine at the Sun Devil Fitness Complex in Tempe on Feb. 2, 2021. Photo by Alex Gould for the State Press
Charting vaccine effectiveness
Researchers measure vaccine effectiveness to evaluate how well a particular vaccine protects a population against infection, symptomatic illness, hospitalization and death under real-world conditions. The current research project aims to accurately estimate the effectiveness of both influenza and COVID-19 vaccines against respiratory virus-associated illness in the context of varying social determinants and observed disparities in health outcome.
In the case of flu vaccines, effectiveness can vary considerably from season to season based on two critical factors: how closely the vaccine matches a circulating seasonal strain of the influenza virus and the characteristics of those vaccinated, including age and health status.
Seasonal flu vaccines are designed to protect against four main groups of flu viruses.
“When the Food and Drug Administration Vaccines and Related Biological Products Advisory Committee makes the final decision about vaccine viruses for domestic flu vaccines, the committee uses a different combination of criteria, such as which flu viruses are making people sick prior to the upcoming season, which flu viruses are spreading prior to the upcoming season, ability of vaccine viruses to confer cross-species protection and how well the viruses in the previous season’s vaccines may protect. That means every year, we need to study how well the current year’s flu vaccine is doing,” says Murugan, who is also the technical and program director of the Biodesign Clinical Testing Laboratory.
The CDC’s efforts are aimed at untangling the skein of data gathered to evaluate vaccine effectiveness over time and across populations of differing age, ethnicity, gender and other factors. To this end, Arizona has been selected as one of seven nationwide CDC-approved Influenza Vaccine Effectiveness Network Centers.
“Biodesign’s strategic partnership with Phoenix Children’s and Valleywise Health will allow us to compile one of the most detailed and comprehensive portraits of vaccine effectiveness ever undertaken,” says Joshua LaBaer, executive director of the Biodesign Institute. “With this data in hand, we can better protect diverse communities at risk, design more effective vaccines and distribution strategies, and ultimately save many lives from the threat of infectious disease.”
A comprehensive approach
The new study has two primary components. The first is designed to measure the effectiveness of influenza and COVID-19 vaccines during the flu season in the U.S. More than 1,000 participants seeking care in ambulatory settings will be enrolled. This component will identify laboratory-confirmed cases of influenza and COVID-19 and estimate vaccine effectiveness.
The project will also collect social data on determinants. Here, the aim is to identify communities disproportionately affected by influenza and COVID-19 infections and plan for the implementation and evaluation of programs to monitor vaccine effectiveness.
Next, the researchers will identify and characterize influenza and SARS-CoV-2 genomic subtypes and variants. The project continues with the ongoing monitoring of annual SARS-CoV-2 and COVID-19 vaccine effectiveness outside of the influenza season, collecting and verifying vaccination data, and reporting the relative effectiveness of different COVID-19 vaccines to the CDC.
Another component of the project will investigate vaccine-induced immune responses over time. Some 250 participants will be enrolled for collection of longitudinal serum specimens, the first on or before vaccination, then months after receipt of influenza and COVID-19 vaccines.
Multiple COVID-19 vaccines and annual flu shots are recommended to mitigate waning immunity over time, though the impact of repeated vaccination on vaccine effectiveness is not yet well understood. To address this, researchers will collect paired blood samples before and after vaccination.
Power in numbers
Children and adults with acute respiratory infection seeking care in outpatient clinics managed by Phoenix Children’s, Valleywise Health Medical Center and ASU Student Health Services will be evaluated. The researchers will collect specimens along with relevant demographic and clinical data for laboratory-confirmed cases of influenza and COVID-19.
Both project components draw heavily on ASU’s close alliance with Phoenix Children’s and Valleywise Health, as well as ASU’s Health Services and the Mirabella on-campus retirement community, allowing for across-the-board testing of vaccine effectiveness on adults, pediatric and elderly patients, and underserved communities who have received influenza and COVID-19 vaccinations.
“This collaboration is an invaluable opportunity to get much-needed clarity on the presence of respiratory viruses circulating within our communities and the effectiveness of currently available vaccines against these viruses,” said Dr. Joanna Kramer, a Phoenix Children’s pediatrician and site principal investigator. “I look forward to sharing study findings with the medical community at large, so children and communities can be as protected as possible.”
Serological studies of collected blood from confirmed influenza and COVID-19 patients will be shared with network laboratories and used to estimate vaccine effectiveness against both diseases and evaluate the public health impact of vaccine programs.
Prior vaccination, infection and other medical health data will be gathered through interviews and electronic health records (EHR) extraction. To ensure reliable and timely monitoring, patients will be able to schedule follow-up testing at home and can even request a bilingual health care provider, as needed.
“The close partnership involving ASU's leadership and technical expertise, and the experienced clinical partners at Valleywise Health and Phoenix Children’s, will make this ambitious project possible,” says Dr. Jeffrey Curtis of Valleywise Health. “We expect to learn more about biological and social factors that make some people respond better to vaccines and about defenses viruses can use to evade vaccine-mediated immunity. I believe this study will lead to better ways to prevent and respond to future epidemics.”
The CDC’s multidisciplinary program provides a comprehensive approach to study vaccine effectiveness and better understand social determinates that may account for health disparities. In addition to safeguarding human health, the findings will have important, long-lasting policy implications for future vaccination efforts.
“The new CDC project plays to many of ASU’s greatest strengths in the health care domain, from our broad expertise in vaccine science to our exceptional clinical testing capacities and the university’s passion for creativity, innovation and service to our communities,” says Sally C. Morton, executive vice president and professor of ASU Knowledge Enterprise.
Multidisciplinary team
The scale and breadth of this project requires a multidisciplinary team of researchers. The team’s clinical experts include Drs. Joanna Kramer with Phoenix Children’s; Jeffrey Curtis with Valleywise Health; and Mario Islas with ASU Health Services. Additional team members include Primary Investigator Vel Murugan; Yunro (Roy) Chung, a biostatistician with ASU College of Health Solutions; Efrem Lim, a virologist with ASU School of Life Sciences; Matthew Scotch, a molecular epidemiologist with ASU College of Health Solutions; Leah Doane and Rick Cruz, health disparity experts from ASU College of Liberal Arts and Sciences; Mitch Magee, an expert in clinical research from ASU School of Life Sciences; and Craig Woods, an expert in clinical site management from the Institute for Future Health.
Top image: Researchers measure vaccine effectiveness to evaluate how well a particular vaccine protects a population against infection, symptomatic illness, hospitalization and death under real-world conditions. The current research project aims to accurately estimate the effectiveness of both influenza and COVID-19 vaccines against respiratory virus-associated illness in the context of varying social determinants and observed disparities in health outcome. Graphic by Shireen Dooling
More Science and technology

ASU to host 2 new 51 Pegasi b Fellows, cementing leadership in exoplanet research
Arizona State University continues its rapid rise in planetary astronomy, welcoming two new 51 Pegasi b Fellows to its exoplanet research team in fall 2025. The Heising-Simons Foundation awarded the…

ASU students win big at homeland security design challenge
By Cynthia GerberArizona State University students took home five prizes — including two first-place victories — from this year’s Designing Actionable Solutions for a Secure Homeland student design…

Swarm science: Oral bacteria move in waves to spread and survive
Swarming behaviors appear everywhere in nature — from schools of fish darting in synchrony to locusts sweeping across landscapes in coordinated waves. On winter evenings, just before dusk, hundreds…