New gas separation method improves sustainability of product production

An illustration inspired by the structure of covalent organic frameworks that Arizona State University researchers Kailong Jin and Jerry Lin are working to develop into membranes. Image courtesy Erika Gronek, ASU/Midjourney
Did you know that the synthetic fibers of your clothing or the plastic packaging of your snacks start as petroleum?
Getting petroleum to a point that it can be used in product production, petroleum refining or in the petrochemical industry all requires a labor-intensive process that uses gases.
These industries need to separate ethylene, propane and xylene isomers, whose molecular sizes are usually less than one nanometer in diameter, which is incredibly small. A millimeter, for example, is made up of one million nanometers. These molecules are conventionally separated from each other by distillation or other energy-intensive thermal processes, but ASU researchers are developing a more energy-efficient, membrane-based molecular separation process.
Kailong Jin leads a research group as an assistant professor of chemical engineering in the School for Engineering of Matter, Transport and Energy, part of the Ira A. Fulton Schools of Engineering at Arizona State University. He is the principal investigator of a National Science Foundation-funded research project to develop membranes with pores smaller than one nanometer, which would separate the molecular gas or vapor mixtures from each other.
Jin’s focus is on developing a nanoporous material for the membranes. The experimental material is a polymer base called a covalent organic framework, sometimes referred to as a COF.
The membranes will reduce thermal energy used and carbon emissions produced during molecular separation.
Covalent organic frameworks are a type of crosslinked polymers that exhibit a crystalline structure; this means they are uniform in their atomic arrangement and stable in their behavior. Crystalline materials often have regular structures that are predictable based on their building blocks, making them ideal for experimentation where other variables will change systematically, in this case the pore size of the membrane. A COF has a regular pore size structure that can be manipulated: tuned from 0.5 nanometers all the way up to 5 nanometers.
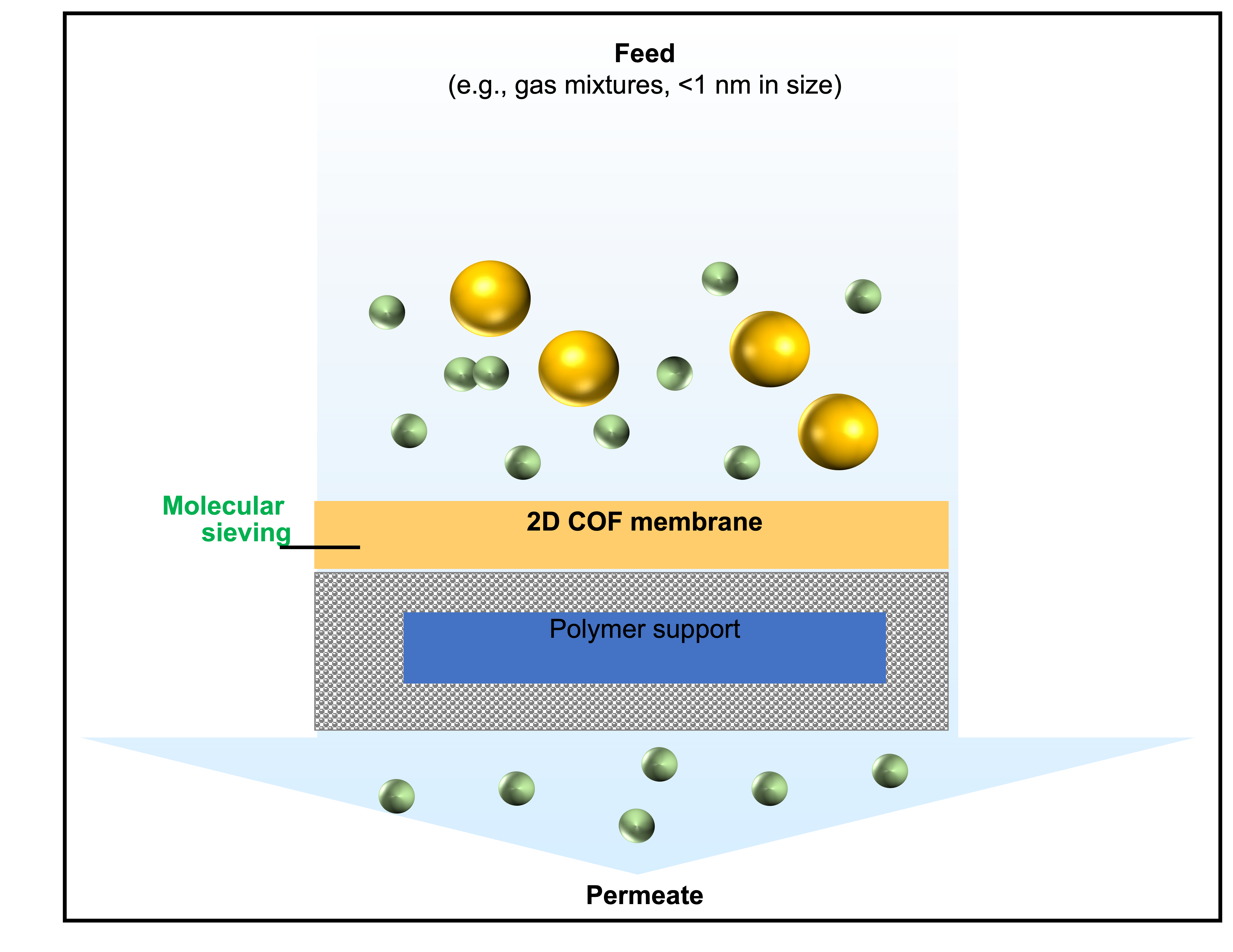
Using different pore sizes, one can separate materials by design. For example, to separate gas molecules that measure 0.5 nanometers from those that measure 0.6 nanometers, the pore size must be tuned to 0.55 nanometers. The molecule that is 0.5 nanometers will pass through the membrane, while the molecule that is 0.6 nanometers will be unable to.
Illustration of the gas separation process, in which gas molecules of the appropriate size pass through the stacked covalent organic framework membrane and its polymer support. Image courtesy Kailong Jin
The challenge of Jin’s work is that the covalent organic framework materials are not easily usable for manufacturing, as they typically come in the form of a powder. In order to separate molecules from each other, the membrane material must be in the form of a sheet that gases are forced through.
Jin’s research group has developed a new method to synthesize these COF membrane sheets. The powdered materials can be first exfoliated and then assembled into a membrane sheet by a scalable filtration coating method. The method disperses and suspends these exfoliated COF sheets in a solution, then uses another filtration process to deposit these COF sheets onto the support substrate, which creates an integrated membrane. Once this integrated membrane is complete, it can separate tiny gas molecules.
ASU Regents Professor Jerry Lin, co-primary investigator of the NSF grant project, will then test the gas separation performance of the synthesized COF membranes. Currently, the research is focused on separating gases ethylene, propane and xylene isomers used in industries such as petroleum refining. Products derived from petrochemicals are used in building materials, paint, packaging, clothing and medical equipment.
The gaseous mixture is separated for downstream chemical reactions or other applications. Once the process is fine-tuned, the membranes could be used in industries far beyond petroleum refining.
Two doctoral students, Richard Nile and Jose Cazares, are now working on a COF-related material for liquid and gas separation research, under the guidance of Jin and Lin. The NSF grant will allow them to add one or two additional student researchers. The added help will expedite the development of their COF membrane, improving the energy efficiency of manufacturing methods as quickly as possible in the race against climate change.
More Science and technology

Diagnosing data corruption
You are in your doctor’s office for your annual physical and you notice the change. This year, your doctor no longer has your…
Large-scale study reveals true impact of ASU VR lab on science education
Students at Arizona State University love the Dreamscape Learn virtual reality biology experiences, and the intense engagement it…

ASU-led space telescope is ready to fly
The Star Planet Activity Research CubeSat, or SPARCS, a small space telescope that will monitor the flares and sunspot activity…