Treatment for autism symptoms earns ASU researchers patent

ASU Professor Rosa Krajmalnik-Brown and Assistant Professor Daewook Kang (now with the University of Toledo) in an ASU file photo from 2017. Krajmalnik-Brown, Kang and Professor James Adams are three of six co-inventors who were awarded a patent for their treatment for autism and related symptoms.
Editor’s note: This story is featured in the 2022 year in review.
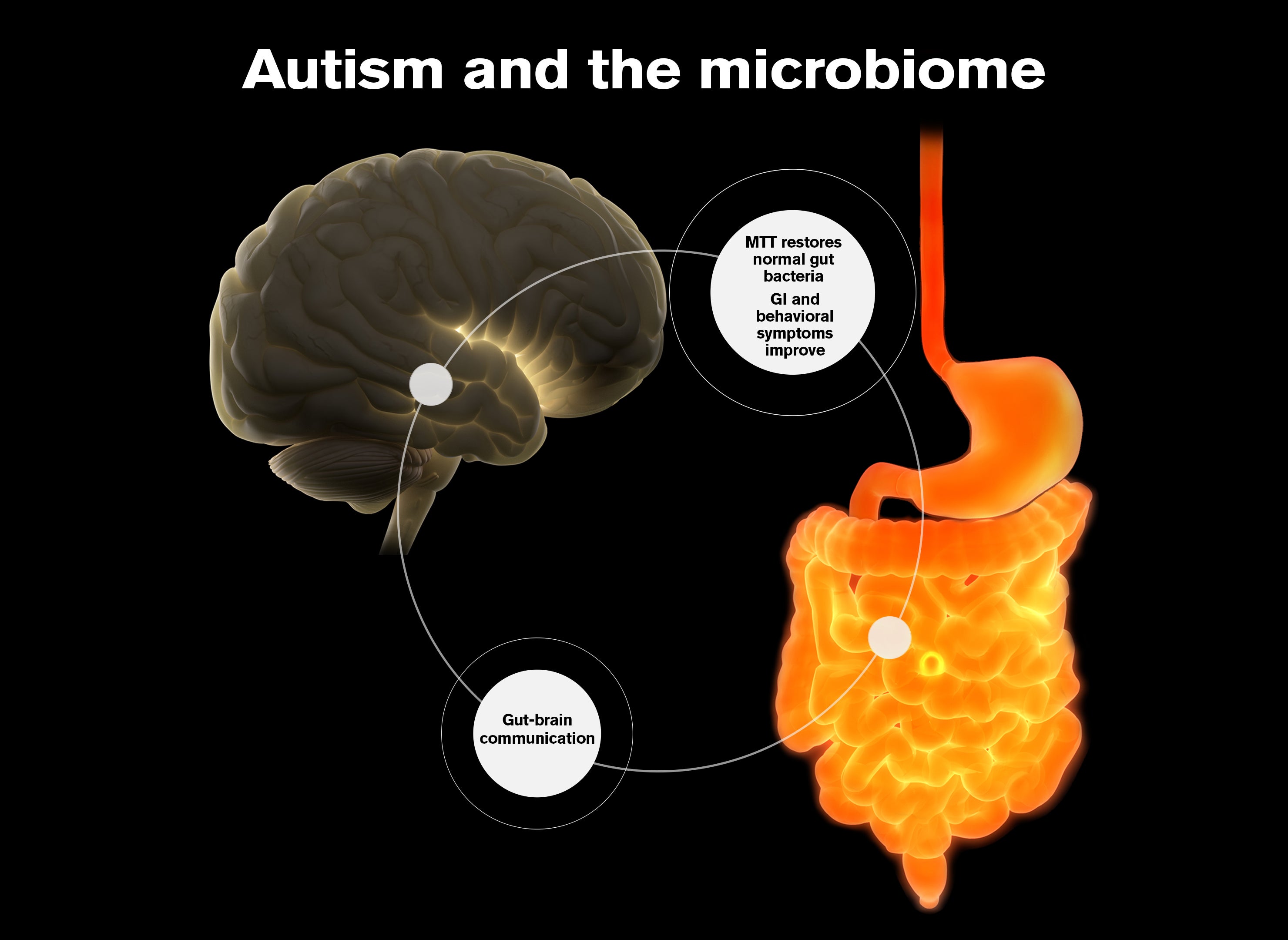
A new treatment for autism, created by Arizona State University researchers and their colleagues, has been granted a patent by the U.S. Patent Office. The therapy, called Microbiota Transplant Therapy (MTT), is aimed at improving chronic gastrointestinal symptoms often associated with the disorder.
Receiving the approval of the patent for this promising treatment is also an important step toward developing a Food and Drug Administration approved medication for treating the core symptoms of autism. An initial study of Microbiota Transplant Therapy suggests that it may be effective in treating both core autism symptoms and chronic gastrointestinal symptoms.
Rosa Krajmalnik-Brown, ASU professor and director of the Biodesign Center for Health Through Microbiomes, and a pioneer in research on the gut microbiome and autism, is a co-inventor of the treatment.
“Our mission at the ASU Biodesign Center for Health Through Microbiomes is to improve human health by developing new methods to modify the microbiome. This is at the core of our center, and it’s exciting that we have a patent to achieve this,” said Krajmalnik-Brown, a professor with the School of Sustainable Engineering and the Built Environment.
Autism now affects one in 44 children in the United States, often presenting major challenges in language, social interactions and behavior. Many people with autism have significant health problems, including 30–50% with chronic gastrointestinal symptoms such as constipation, diarrhea and abdominal pain. These symptoms are difficult to treat and can persist for many years or even decades. Chronic pain caused by gastrointestinal distress can worsen other symptoms associated with autism, including irritability, attention deficits, behavioral issues and sleep disturbances.
“We are excited about this patent approval. Our open-label pilot study and two-year follow-up study found major improvements in gastrointestinal and autism symptoms. Now, we are conducting randomized clinical trials to fully evaluate the efficacy of treatment with MTT,” said co-inventor James Adams. Adams is a professor with the School for Engineering of Matter, Transport and Energy, one of the seven Ira A. Fulton Schools of Engineering at ASU.
James Adams
In 2017, the ASU researchers and their collaborators developed MTT to improve gastrointestinal symptoms. MTT involves pre-treatment with a special antibiotic to eliminate harmful bacteria, a bowel cleanse to remove remaining bacteria, and purified intestinal microbiota from healthy, carefully screened human donors.
The treatment, similar to the fecal transplant commonly used to treat intestinal infections such as C. Difficile, involves 10 weeks of intense daily therapy. The approach is based on pioneering research by Dr. Thomas Borody with the Centre for Digestive Diseases in Australia, who first used this method to successfully treat his patients with autism, and Professors Alex Khoruts and Michael Sadowsky at the University of Minnesota, who developed the methods for producing purified microbiota.
In their 2017 study, Adams, Krajmalnik-Brown and their research team found that MTT reduced gastrointestinal symptoms by approximately 80%, and initially reduced autism symptoms by about 25%. But, as an open-label study, they observed some placebo-effect. A follow-up study of all 18 participants two years after the treatment was completed found that most continued to see a considerable improvement in gastrointestinal symptoms. Additionally, an expert autism evaluator reported a nearly 50% reduction in core autism symptoms.
Measurements of the participants’ microbiota at Krajmalnik-Brown’s lab by Assistant Professor Daewook Kang (now with the University of Toledo) showed that although the children, aged 7 to 16 at the time of the study, initially had a low diversity of gut bacteria, the diversity and presence of beneficial microbes had increased and improved in the two years following the treatment. Based on these promising results, the FDA granted “fast track” status for MTT in 2019, which means a rapid review and more assistance from the FDA.
Recent research suggests our gut microbiomes affect brain communication and neurological health. Worldwide, interest is growing in the idea that changes in normal gut microbiota may be responsible for triggering various conditions. At ASU, a research team is exploring using the microbiome to treat autism symptoms. Image by Shireen Dooling
Approval of the patent is also important because pharmaceutical companies can invest in conducting Phase 2 and Phase 3 clinical trials, which are required for FDA approval and release of the treatment to the public. The patent has been licensed to Finch Therapeutics, which is planning clinical trials in mid-2022.
Meanwhile, the ASU team is continuing its own Phase 2 clinical trials for adults with autism (the final participants have started treatment) and children with autism (half of the participants have started treatment). These studies will be important for ultimately winning FDA approval.
Information on the patent, as well as a full list of collaborators, can be found on the U.S. Patent Office website (U.S. Patent number 11202808).
More Science and technology

ASU to host 2 new 51 Pegasi b Fellows, cementing leadership in exoplanet research
Arizona State University continues its rapid rise in planetary astronomy, welcoming two new 51 Pegasi b Fellows to its exoplanet…

ASU students win big at homeland security design challenge
By Cynthia GerberArizona State University students took home five prizes — including two first-place victories — from this year’s…

Swarm science: Oral bacteria move in waves to spread and survive
Swarming behaviors appear everywhere in nature — from schools of fish darting in synchrony to locusts sweeping across landscapes…