New microfluidic device minimizes loss of high value samples

Alexandra Ros, a professor in ASU’s School of Molecular Sciences and the Center for Applied Structural Discovery in the Biodesign Institute. Photo credit: Mary Zhu
A major collaborative effort that has been developing over the last three years between Arizona State University and European scientists has resulted in a significant technical advance in X-ray crystallographic sample strategies.
The ASU contribution comes from the School of Molecular Sciences , Department of Physics and the Biodesign Institute Center for Applied Structural Discovery.
The European X-ray Free Electron Laser (EuXFEL) is a research facility of superlatives: It generates ultrashort X-ray pulses — 27,000 times per second and with a brilliance that is a billion times higher than that of the best conventional X-ray radiation sources. After 10 years of construction, it opened for initial experiments in late 2017. The group of Alexandra Ros, professor in ASU’s School of Molecular Sciences, was awarded the second allocation of beam time amongst worldwide competitors.
Their results, published Sept. 9 in Nature Communications, validated a unique microfluidic droplet generator for reducing sample size as well as waste (which can be as high as 99%) in her team’s Serial Femtosecond Crystallography (SFX) experiments. Using this, they determined the crystal structure of the enzyme 3-Deoxy-d-manno-Octulosonate 8-Phosphate Synthase (KDO8PS) and revealed new detail in a previously undefined loop region of the enzyme that is a potential target for antibiotic studies.
“We are excited that this work, resulting from a huge collaborative effort, has been well received in the XFEL community,” Ros said. “We are further developing this method and are seeking synchronization of the microfluidic droplets with the pulses of XFELs. At this very moment, a small team of ASU students has just finished performing experiments at the Linac Coherent Light Source at the SLAC National Accelerator Laboratory in Menlo Park, California, to refine the method. There could not have been better timing for the publication of our work.”
SLAC has been the XFEL facility best known to U.S. scientists, where the now-famous work on crystallography of protein nanocrystals — by the ASU team led by professors John Spence and Petra Fromme — was carried out. SLAC and its companion in Europe, also at Hamburg, have been very successful and consequently, have become heavily overbooked. The coming-on-line of the new facility, with its giant 2.6-mile accelerator tunnel and atomic length scale resolution, has relieved some of the demand on the other facilities, while offering grand new possibilities in the physical sciences.

The ASU team with EuXFEL beam line scientists during one of the designated shifts. The experimental hutch is behind the team. From left: Nadia Zatsepin (ASU), Jorvani Cruz Villarreal (ASU), Patrik Vagovič (EuXFEL), Petra Fromme (ASU), Jose Meza Aguilar (ASU), Alexandra Ros (ASU), Jesse Coe (ASU), Ana Egatz Gomez (ASU), Gerrit Brehm (ASU/Georg August Univeristy Goettingen), Austin Eichelmeyer (ASU), Richard Kirian (ASU), Richard Bean (EuXFEL), Marc Messerschmidt (EuXFEL), Katherina Doerner (EuXFEL).
SFX is a promising technique for protein structure determination, where a liquid stream containing protein crystals is intersected with a high-intensity XFEL beam that is a billion times brighter than traditional synchrotron X-ray sources.
Although the crystals are destroyed by the intense XFEL beam immediately after they have diffracted, the diffraction information can, remarkably, still be recorded thanks to the state-of-the-art detectors. Powerful new data analysis methods have been developed, allowing a team to analyze these diffraction patterns and obtain electron density maps and detailed structural information of proteins.
The method is specifically appealing for hard-to-crystallize proteins, such as membrane proteins, as it yields high-resolution structural information from micro- and even nanocrystals, thus reducing the contribution of crystal defects and avoiding the tedious, if not impossible, growth of the large crystals demanded by traditional synchrotron-based crystallography.
While crystallography with XFELs has been a powerful technique for unraveling the structures of large protein complexes and also permitting time-resolved crystallography, this cutting-edge science nevertheless engenders a major problem. Because of the small “hit” rate it requires huge amounts of suspended protein, which although not irradiated, are cumbersome to retrieve for most protein samples. As much as 99% of the protein can be wasted.
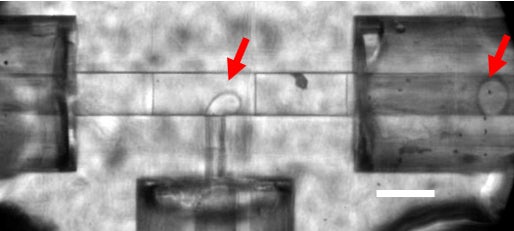
Droplets are generated in the microfluidic T-junction, indicated by red arrows. The droplet carries crystals that will be delivered to the XFEL.
Herein lies the major technical advance made by Ros and her team. They have developed a 3D-printed microfluidic device, which is high-resolution, and generates aqueous-in-oil droplets of variable droplet segmentation that can be synchronized to the free electron laser pulses. This dramatically reduces the amount of purified protein needed for the European XFEL experiment from the currently typical (and almost inaccessible) 1 g requirement for full data set recording.
The researchers’ approach interleaves sample-laden liquid “slugs” within a sacrificial liquid, so that a fast-moving liquid microjet is maintained with sample present only during exposure to the femtosecond XFEL pulses (one millionth of one billionth of a second in duration).
The team of scientists has demonstrated droplet generation of the enzyme KDO8PS crystal suspensions with the microfluidic droplet generator and shown that the droplet generation frequency can be controlled by the rates of the aqueous and oil streams. The diffraction quality of the crystals of KDO8PS is similar both when injected in aqueous droplets surrounded by oil or by continuous injection with a gas dynamic virtual nozzle, with approximately 60% reduction in sample consumption achieved with droplet injection.
The determined structure revealed new detail in a previously undefined loop region of KDO8PS, a potential target for antibiotic studies. These results advocate for future routine integration of droplet generation by segmented oil flow at other XFELs around the world.
Co-first authors on this paper are Ros’ doctoral students Austin Echelmeier (graduated) and Jorvani Cruz Villarreal in the School of Molecular Sciences.
More Science and technology

Will this antibiotic work? ASU scientists develop rapid bacterial tests
Bacteria multiply at an astonishing rate, sometimes doubling in number in under four minutes. Imagine a doctor faced with a…

ASU researcher part of team discovering ways to fight drug-resistant bacteria
A new study published in the Science Advances journal featuring Arizona State University researchers has found…

ASU student researchers get early, hands-on experience in engineering research
Using computer science to aid endangered species reintroduction, enhance software engineering education and improve semiconductor…