ASU researchers provide a golden opportunity to improve cancer treatment accuracy

Subhadeep Dutta, a chemistry doctoral student at Arizona State University, contributes to an interdisciplinary research group led by Kaushal Rege, a professor of chemical engineering in the Ira A. Fulton Schools of Engineering at ASU, to develop a quicker, cheaper and more user-friendly way to measure cancer radiation treatment doses. Photo by Erika Gronek/ASU
Accuracy is extremely important for cancer radiation treatments. Today, more than half of people diagnosed with cancer undergo radiotherapy, where a precise total dose of ionizing radiation is delivered over multiple treatment sessions to effectively stop the growth of cancer cells.
It is critical to monitor radiation doses in real time to ensure a person receives the exact prescribed dose. When overexposed to radiation, treatment can become harmful — damaging healthy cells and inducing toxicity that can cause future negative consequences. Underexposure, on the other hand, means cancer cells or a tumor may not be effectively treated.
Even with quality assurance practices, equipment issues or a patient moving too much during treatment can affect how much radiation the patient receives.
Dr. Stephen Sapareto, former director of medical physics at the Gilbert, Arizona, Banner MD Anderson Cancer Center, was concerned about existing expensive, slow and difficult-to-use methods to measure radiation doses. He approached Kaushal Rege, a professor of chemical engineering in the Ira A. Fulton Schools of Engineering at Arizona State University.
“They wanted something that is easier to use and more cost-effective compared to existing solutions,” said Subhadeep Dutta, a chemistry doctoral student who works on an interdisciplinary research team in the Rege Bioengineering Lab.
Interdisciplinary teams solve interdisciplinary problems
Problems of this nature can’t be solved by one discipline, so it’s important to have a diverse research team working on the solution. Rege’s lab incorporates chemical and biomedical engineers in addition to biology and chemistry researchers, like Dutta. Rege regularly collaborates with students from other schools at ASU.
"These synergistic interactions have led to the development of new technologies for addressing cutting-edge biomedical problems," Rege said.
Dutta brings an extensive background in organic chemistry, which can inform how chemicals react to radiation.
"This research project is based on new developments in fundamental nanochemistry applied to solving a clinically relevant problem," Rege said. "Subhadeep's background in chemistry is important for developing the fundamental underpinnings of the technology while keeping the eventual biomedical application in focus."
When he came to ASU to earn his doctoral degree in chemistry, Dutta was eager for the opportunity to use his chemistry background to work on applied projects.
“I always wanted to do something in the application side of chemistry, and that’s why I moved to ASU and became part of (Dr. Rege’s) research team,” Dutta said. “We all have these different types of expertise, and if I have a question that I might not know, somebody else in our group can help me solve it.”
Chemistry colors radiation treatment accuracy
With input from collaborators at Banner-MD Anderson, Dutta and other researchers from Rege’s lab, including recently graduated student Karthik Pushpavanam, set out to create a new measurement method that is safe for people receiving radiotherapy, as well as quicker, cheaper and more user-friendly.
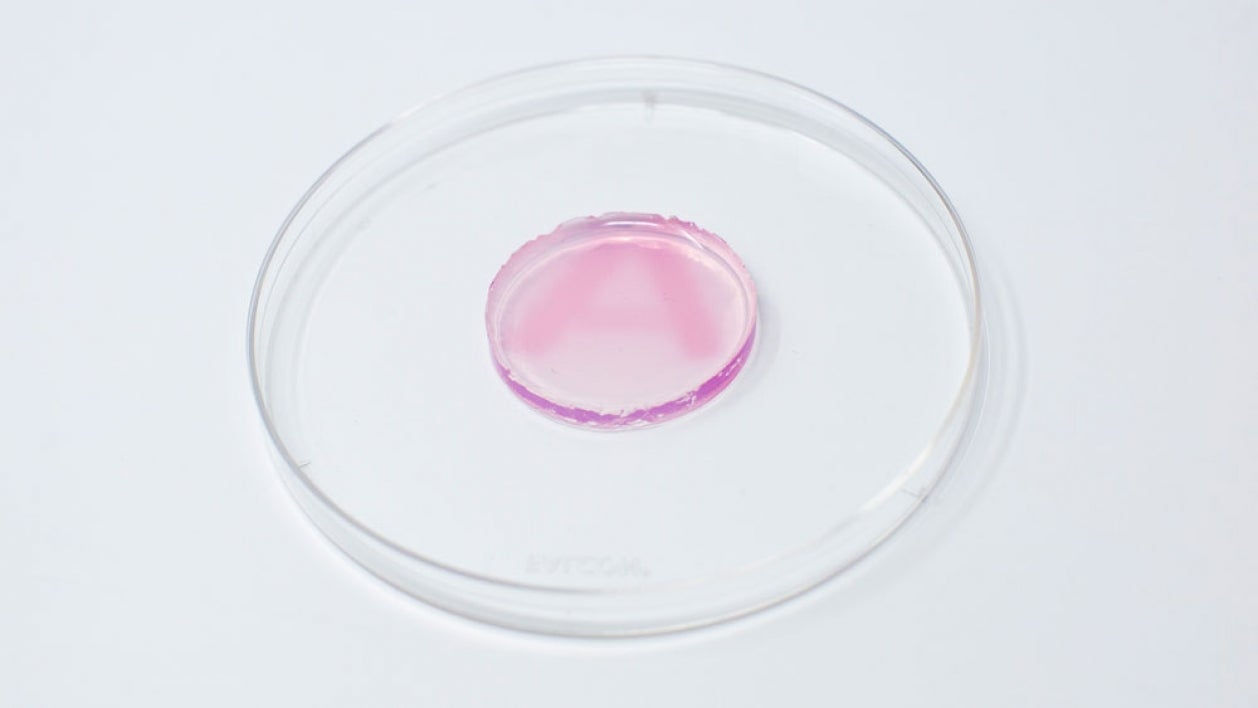
Gold nanoparticles are well understood in chemistry literature for changing color based on size, shape and molecular interactions.
“Gold is a good fit because it can change colors based on its size and shape in the nanoparticle form,” Dutta said. “As soon as radiation hits the colorless gel (containing gold ions), it starts to make nanoparticles, and a color change can be observed within 15 minutes.”
This type of gel, called a hydrogel, is typically made of 90% water. A patch of the hydrogel can be placed on a person’s skin at the site where radiation will be directed. As radiation passes through the hydrogel, it splits the water molecules into reducing agents (e.g., electrons) and starts the chemical reaction that turns the gold salts into nanoparticles, giving color to the gel. In this case, the nanoparticles turn pink/maroon.
The intensity of the color in the gel can be correlated to a measurement of the delivered radiation dose. So, within minutes, clinicians can use the gel to calculate how much radiation the person has received and adjust future doses if it differs from that session’s prescribed dose. For example, if a person was supposed to receive 1 unit of radiation but instead received 1.3 units, the next session could be adjusted to even out the cumulative dose.
So far, the team has been able to successfully demonstrate the gold nanoparticles are effective at measuring radiation doses using clinical systems at Banner-MD Anderson and Arizona Veterinary Oncology, and they’ll soon use the method more widely.
In the project’s next phase, Dutta and the research team are planning to develop a companion app. By taking a photo of the colored gel, the app would assess the color and provide the correlated dose measurement in seconds, making the technology even more user-friendly. This can have further implications for radiation safety and security.
More Science and technology

ASU and Deca Technologies selected to lead $100M SHIELD USA project to strengthen U.S. semiconductor packaging capabilities
The National Institute of Standards and Technology — part of the U.S. Department of Commerce — announced today that it plans to…
From food crops to cancer clinics: Lessons in extermination resistance
Just as crop-devouring insects evolve to resist pesticides, cancer cells can increase their lethality by developing resistance to…

ASU professor wins NIH Director’s New Innovator Award for research linking gene function to brain structure
Life experiences alter us in many ways, including how we act and our mental and physical health. What we go through can even…