7-year School of Molecular Sciences collaboration bears new fruit

Rebekka Wachter (right) and Marcia Levitus have been collaborating for seven years.
In an academic field where female researchers comprise a talented minority, two professors from Arizona State University's School of Molecular Sciences are making an impact. Rebekka Wachter and Marcia Levitus have been collaborating for seven years, with several co-authored, highly rated publications to their credit.
Wachter is an expert in the field of structural characterization of proteins. Her team is interested in understanding how a protein’s three-dimensional structure, dynamic motions and self-assembly guide its biological function.
Levitus’ expertise lies in the field of single-molecule spectroscopy. Her research group focuses on the development and application of state-of-the-art techniques for single-molecule detection to study complex biological systems.
Their current research involves an elegant study, which has just been published in the Journal of Biological Chemistry.
It involves the carbon-fixing activity of the most abundant enzyme in nature, Rubisco, which carries out the vast majority of carbon dioxide fixation reactions on Earth and is therefore extensively regulated.
Carbon fixation is the process in plants and algae by which atmospheric carbon dioxide is converted into organic carbon compounds, such as carbohydrates, usually by photosynthesis.
Under high irradiance, Rubisco activase (Rca), another enzyme, provides continuous support of Rubisco activity. In fact, without this activity, higher plants (e.g. crops) would perish. Under rapidly changing light conditions, Rca assists in balancing the light and dark reactions of photosynthesis. The catalytic roles of higher plant Rca played by various sub units has remained an enigma, a puzzle which is addressed in the current paper.
In 2012, the team established fluorescence correlation spectroscopy (FCS) protocols to estimate the oligomeric composition of Rca as a function of subunit concentration.
Fluorescence Correlation Spectroscopy is based on the analysis of the fluctuations in the fluorescent signal of a small number of molecules. Correlation analysis of the fluorescence fluctuations yields kinetic information about the dynamic processes that cause the changes in the fluorescent signal.
“It is difficult to find one technique that has the ability to monitor the entire range from small oligomers to very large protein aggregates,” Wachter explained. "Our FCS approach manages to do this and has allowed us to come up with a novel activity model that involves assembly and disassembly of subunits. Currently, this is the only model that fits all the available experimental data.”
Adenosine triphosphate, or ATP for short, is the energy currency of life. ATP is a high-energy molecule found in every cell. Its job is to store and supply the cell with needed energy. ATPase is an enzyme. ATPase breaks down ATP into adenosine diphosphate and free phosphate ion, releasing energy.
After analysis of mountains of data, the team has proposed that dynamic assembly–disassembly is part of the ATPase cycle. According to this model, the association of dimers with tetramers generates a hexamer that forms a closed ring at high ATP and magnesium levels. Upon hydrolysis and product release, the toroid breaks open and dissociates — the Rca falls off Rubisco to form a dimer and tetramer which then reassembles in solution.
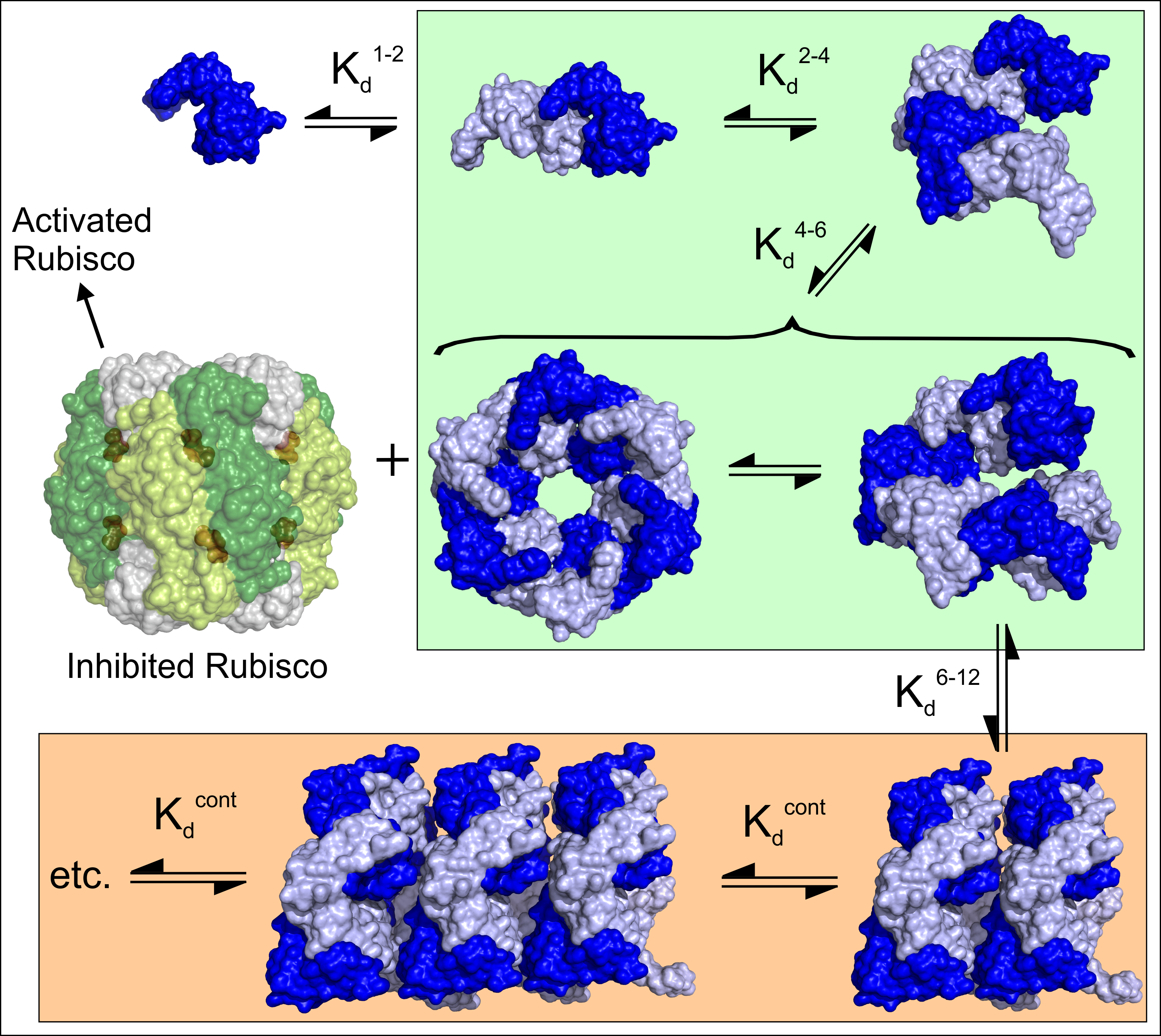
The assembly-disassembly is highly regulated and only observed with higher plant Rubisco activase (in this study from tobacco). Anything larger than a hexamer is catalytically inactive. Plants move Rca into an inactive state (spiral assemblies) when Rca is not needed. Magnesium binding reactivates the Rca. The data from the team’s FCS and ATPase activity shows clear and clean evidence that anything greater than a hexamer is definitely inactive.
"These techniques are convenient ways to study molecular diffusion and oligomerization without physical perturbation of the sample in a wide range of concentrations. Our results show clear evidence of the coexistence of multiple oligomerization states, and allowed us to propose the assembly-disassembly mechanism for Rubisco activase," said Levitus, who is also part of the Center of Single Molecule Biophysics in ASU's Biodesign Institute. "In addition, the methodology that we developed in this work can be broadly used to investigate self-association in other proteins."
This research was supported by a grant from the Department of Energy, Basic Energy Sciences.
More Science and technology

ASU technical innovation enables more reliable and less expensive electricity
Growing demand for electricity is pushing the energy sector to innovate faster and deploy more resources to keep the lights on…

What do a spacecraft, a skeleton and an asteroid have in common? This ASU professor
NASA’s Lucy spacecraft will probe an asteroid as it flys by it on Sunday — one with a connection to the mission name.The asteroid…

Hack like you 'meme' it
What do pepperoni pizza, cat memes and an online dojo have in common?It turns out, these are all essential elements of a great…

