Physicist joins ASU LightWorks to help solarize society

Ivan Ermanoski says he came to ASU because of its commitment to improve the world’s future and because of ASU’s multidisciplinary research environment. He says he values working with people outside of his area of expertise, something “that comes easily in the ASU environment.” Photo courtesy of Sandia National Laboratories
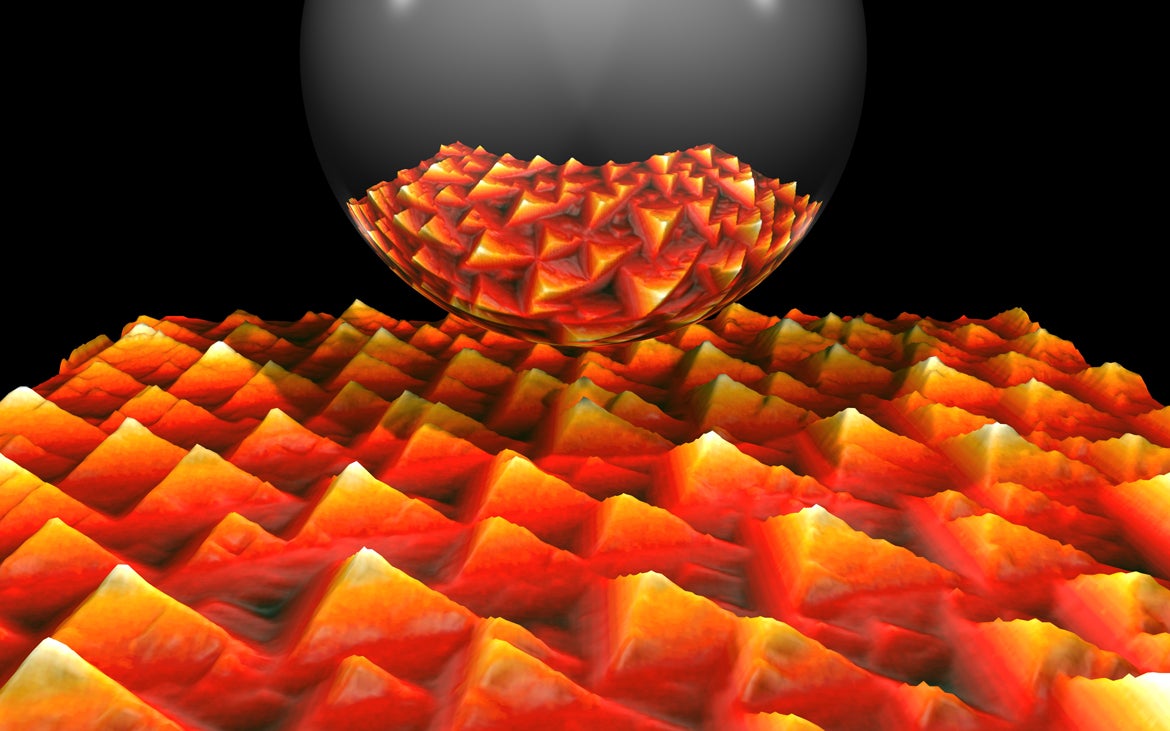
Ivan Ermanoski slips his credit card from his wallet and places it on the table in front of him. The card displays his name, the requisite 16-digit number and an eye-catching scarlet pyramid-patterned background, that of a nano-sized piece of iridium after it was exposed to oxygen. Ermanoski, a physicist, captured the image using a scanning tunneling microscope.
“This is an actual experimental image,” he said. And an inspirational one — he plans to hang a large print of it in his new office at Arizona State University.
The Macedonian-born Ermanoski concentrates on making fuels and products using solar heat. He’s a recent arrival at ASU LightWorks, where he’ll be working on solarizing our society — that is, reducing the use of fossil fuels by replacing them with solar-derived fuels.
To accomplish this, he and his colleagues are planning to use a thermochemical cycle that would keep carbon dioxide from being added to the atmosphere.
The thermochemical cycle begins when a metal oxide is heated until it gives up some of its oxygen. At lower temperatures, the material wants that oxygen restored, and if exposed to carbon dioxide or steam, the material will take an oxygen from those molecules to yield carbon monoxide or hydrogen.
The reaction sequence can be indefinitely repeated, creating a thermochemical loop. The resulting molecules are both energy-rich and can be reacted with one another (in a separate process) to form more conventional hydrocarbon fuels, such as jet fuel, gasoline or diesel.
“This is just the beginning of what we call solar chemistry,” said Ermanoski, who will design the thermochemical reactor within which the thermochemical cycle will occur.
People don’t necessarily appreciate how many goods are produced using fossil fuels, Ermanoski said.
When people think of fossil fuels and the environment, they often think of the effects of burning coal, natural gas or petroleum to get from one place to another or to heat a house or to cook a meal.
But Ermanoski says fossil fuels are everywhere. They’re used in the manufacturing of all sorts of items: fabrics, pens, deodorants, contact lenses, lipstick, clothing, cleansers, medicines, furniture, house paint and even food.
“Most people don’t know that our food system could not survive without fossil fuel input,” Ermanoski said.
He explains that natural gas serves as a source of hydrogen, which is combined with nitrogen to produce ammonia, the foundation of nitrogen fertilizer.
Pictured here is a nano-sized piece of iridium after it was exposed to oxygen. Ermanoski captured the image using a scanning tunneling microscope. He says his work with the transition metal will bear on his research at ASU and has already inspired ideas that he and his current collaborators are working on. Photo by Ivan Ermanoski
At ASU, Ermanoski will be working closely with Ellen Stechel, co-director of LightWorks and an expert in solar thermochemistry, and Jim Miller, a chemical engineer, also a recent arrival to LightWorks. All three scientists previously worked together at Sandia National Laboratories, so they’re familiar with each other’s research style.
“My ideas are usually high level,” Ermanoski said. “I solve a problem, and then I talk with Jim and Ellen, and they tell me how my idea needs to change to be applicable to the world. So, they appreciate my creativity, and I appreciate their deep knowledge of the real world.”
In the real world, the iridium Ermanoski has used in his research is not a particularly practical substance, given its expense. So, it’s used chiefly in scientific models. But that doesn’t mean the time Ermanoski spent working with it was for naught. He says his work with this precious transition metal will bear on his research here, as it involved the study of chemisorption of oxygen on surfaces. In fact, he confides, it has already inspired ideas that he and his collaborators are now working on.
He also confides that he and Miller have different interpretations of what’s hard and what’s easy.
“What I think is hard, he thinks is easy, and what I think is easy he thinks is hard. I think more than anything else, we have overlapping skill sets.”
These will be indispensable in coming up with solutions to this bold endeavor.
“We are very excited that Ivan is joining us in LightWorks and for the opportunity to build out a comprehensive program based on solar thermochemistry — for fuels, industrial and agricultural chemicals, as well as energy storage and production of clean water,” Stechel said.
“This is actually the third time I am hiring Ivan — first as a postdoc, then as staff at Sandia National Labs and now as faculty in LightWorks,” she said. “Needless to say, he has impressed me with his intelligence, his creativity, his out-of-the-box wild ideas and his willingness to come back down to Earth to produce something practical.”
“When you go to physics school, you learn two things. One, the principles of how this world operates, as in, don’t be stupid,” said Ermanoski with a soft laugh. “And two, you really learn how to solve problems. This is important because oftentimes we don’t solve problems.”
Or more precisely, we don’t always solve the real problem, he said. “But when you’re a physicist, you ask, what’s the real problem here?”
For example, Ermanoski cites automakers’ decades-long quest to raise mileage per gallon to reduce the use of gasoline. This was a good idea, he acknowledges, and still is. But is miles per gallon the real problem today? If we’re trying to get from point A to point B, do we even need a car? Is efficient and reliable public transportation a better bet? Or perhaps a technological solution like Skype negates the need to hit the road. What’s more, what are our cities going to look like in 100 years? Are cars still going to serve as a viable way to get around?
“It’s going to change gradually, like things do,” Ermanoski said. “People might think, ‘How can we do without cars?’ If you told people in the year 1900 that they were not going to ride horses and use carriages on a day-to-day basis, they’d probably say, ‘You’re nuts.’ But the reality is the world looks nothing like it did in 1900, and it’s foolish to think that we’re going to be driving cars 100 years from now.”
It might also be foolish to think that solarizing our society will be simple or easy. But Ermanoski doesn’t think that. He’s well aware of the challenges ahead.
“Sometimes ideas are bad because we don’t know how to put them into practice, but then later that may change with new information,” he said. “I really believe in progress. If anything, I’m a daily pessimist, but I’m a long-term optimist.”
More Science and technology

4 ASU researchers named senior members of the National Academy of Inventors
The National Academy of Inventors recently named four Arizona State University researchers as senior members to the prestigious…

Transforming Arizona’s highways for a smoother drive
Imagine you’re driving down a smooth stretch of road. Your tires have firm traction. There are no potholes you need to swerve to…

The Sun Devil who revolutionized kitty litter
If you have a cat, there’s a good chance you’re benefiting from the work of an Arizona State University alumna. In honor of…