ASU scientists unveil a hidden secret of the immortality enzyme telomerase

This figure depicts the enzyme telomerase as well as telomeres relative to a chromosome.
Can we stay young forever, or even recapture lost youth?
Research from the laboratory of Professor Julian Chen in the School of Molecular Sciences at Arizona State University recently uncovered a crucial step in the telomerase enzyme catalytic cycle. This catalytic cycle determines the ability of the human telomerase enzyme to synthesize DNA “repeats” (specific DNA segments of six nucleotides) onto chromosome ends, and so afford immortality in cells. Understanding the underlying mechanism of telomerase action offers new avenues toward effective anti-aging therapeutics.
Typical human cells are mortal and cannot forever renew themselves. As demonstrated by Leonard Hayflick a half-century ago, human cells have a limited replicative lifespan, with older cells reaching this limit sooner than younger cells. This "Hayflick limit" of cellular lifespan is directly related to the number of unique DNA repeats found at the ends of the genetic material-bearing chromosomes. These DNA repeats are part of the protective capping structures, termed "telomeres," which safeguard the ends of chromosomes from unwanted and unwarranted DNA rearrangements that destabilize the genome.
Each time the cell divides, the telomeric DNA shrinks and will eventually fail to secure the chromosome ends. This continuous reduction of telomere length functions as a "molecular clock" that counts down to the end of cell growth. The diminished ability for cells to grow is strongly associated with the aging process, with the reduced cell population directly contributing to weakness, illness, and organ failure.
The fountain of youth at molecular level
Counteracting the telomere shrinking process is the enzyme, telomerase, that uniquely holds the key to delaying or even reversing the cellular aging process. Telomerase offsets cellular aging by lengthening the telomeres, adding back lost DNA repeats to add time onto the molecular clock countdown, effectively extending the lifespan of the cell. Telomerase lengthens telomeres by repeatedly synthesizing very short DNA repeats of six nucleotides — the building blocks of DNA — with the sequence "GGTTAG" onto the chromosome ends from an RNA template located within the enzyme itself. However, the activity of the telomerase enzyme is insufficient to completely restore the lost telomeric DNA repeats, nor to stop cellular aging.
The gradual shrinking of telomeres negatively affects the replicative capacity of human adult stem cells, the cells that restore damaged tissues and/or replenish aging organs in our bodies. The activity of telomerase in adult stem cells merely slows down the countdown of the molecular clock and does not completely immortalize these cells. Therefore, adult stem cells become exhausted in aged individuals due to telomere length shortening that results in increased healing times and organ tissue degradation from inadequate cell populations.
Tapping the full potential of telomerase
Understanding the regulation and limitation of the telomerase enzyme holds the promise of reversing telomere shortening and cellular aging with the potential to extend human lifespan and improve the health and wellness of elderly individuals. Research from the laboratory of Chen and his colleagues, Yinnan Chen, Joshua Podlevsky and Dhenugen Logeswaran, recently uncovered a crucial step in the telomerase catalytic cycle that limits the ability of telomerase to synthesize telomeric DNA repeats onto chromosome ends.
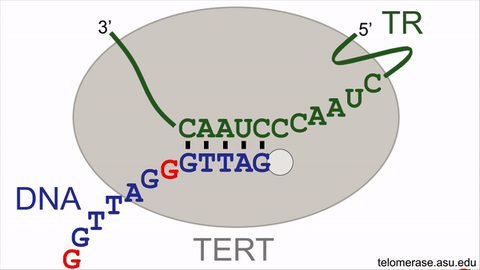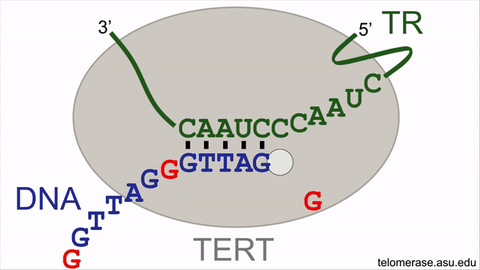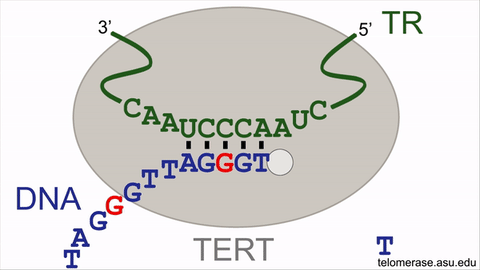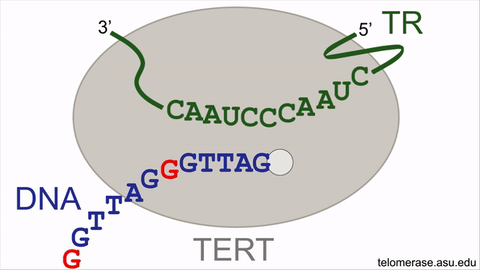
An animation for the telomerase enzyme reaction cycle.
"Telomerase has a built-in braking system to ensure precise synthesis of correct telomeric DNA repeats. This safe-guarding brake, however, also limits the overall activity of the telomerase enzyme," said Professor Chen. "Finding a way to properly release the brakes on the telomerase enzyme has the potential to restore the lost telomere length of adult stem cells and to even reverse cellular aging itself."
This intrinsic brake of telomerase refers to a pause signal, encoded within the RNA template of telomerase itself, for the enzyme to stop DNA synthesis at the end of the sequence ‘GGTTAG’. When telomerase restarts DNA synthesis for the next DNA repeat, this pause signal is still active and limits DNA synthesis. Moreover, the revelation of the braking system finally solves the decades-old mystery of why a single, specific nucleotide stimulates telomerase activity. By specifically targeting the pause signal that prevents restarting DNA repeat synthesis, telomerase enzymatic function can be supercharged to better stave off telomere length reduction, with the potential to rejuvenate aging human adult stem cells.
Human diseases that include dyskeratosis congenita, aplastic anemia, and idiopathic pulmonary fibrosis have been genetically linked to mutations that negatively affect telomerase activity and/or accelerate the loss of telomere length. This accelerated telomere shortening closely resembles premature aging with increased organ deterioration and a shortened patient lifespan from critically insufficient cell populations. Increasing telomerase activity is the seemingly most promising means of treating these diseases.
While increased telomerase activity could bring youth to aging cells and cure premature aging-like diseases, too much of a good thing can be damaging for the individual. Just as youthful stem cells use telomerase to offset telomere length loss, cancer cells employ telomerase to maintain their aberrant and destructive growth. Augmenting and regulating telomerase function will have to be performed with precision, walking a narrow line between cell rejuvenation and a heightened risk for cancer development.
Distinct from human stem cells, somatic cells constitute the vast majority of the cells in the human body and lack telomerase activity. The telomerase deficiency of human somatic cells reduces the risk of cancer development, as telomerase fuels uncontrolled cancer cell growth. Therefore, drugs that increase telomerase activity indiscriminately in all cell types are not desired. Toward the goal of precisely augmenting telomerase activity selectively within adult stem cells, this discovery reveals the crucial step in telomerase catalytic cycle as an important new drug target. Small molecule drugs can be screened or designed to increase telomerase activity exclusively within stem cells for disease treatment as well as anti-aging therapies without increasing the risk of cancer.
Publication: Chen, Y., J.D. Podlevsky, D. Logeswaran and J.J.-L. Chen (2018). A single nucleotide incorporation step limits human telomerase repeat addition activity. EMBO. J. 37: e97953, DOI 10.15252/emboj.201797953.
Grant: This work was supported by National Institutes of Health (NIH), grant number R01GM094450.
More Science and technology

ASU professor breeds new tomato variety, the 'Desert Dew'
In an era defined by climate volatility and resource scarcity, researchers are developing crops that can survive — and thrive —…

Science meets play: ASU researcher makes developmental science hands-on for families
On a Friday morning at the Edna Vihel Arts Center in Tempe, toddlers dip paint brushes into bright colors, decorating paper…

ASU water polo player defends the goal — and our data
Marie Rudasics is the last line of defense.Six players advance across the pool with a single objective in mind: making sure that…

