A tale of two studies: ASU researchers uncover mechanisms of lizard tail regeneration

A green anole lizard (Anolis carolinensis). Photo: Kenro Kusumi
With the hope that someday scientists will advance regenerative therapy in humans, an interdisciplinary team of researchers from Arizona State University and two other institutions has discovered important new clues in exactly how lizards regenerate their tails.
The findings appear in a pair of studies published in a Jan. 15 special issue of the journal “Developmental Biology” that focused on regeneration. The University of Arizona College of Medicine-Phoenix and Victor Chang Cardiac Research Institute also participated in the studies.
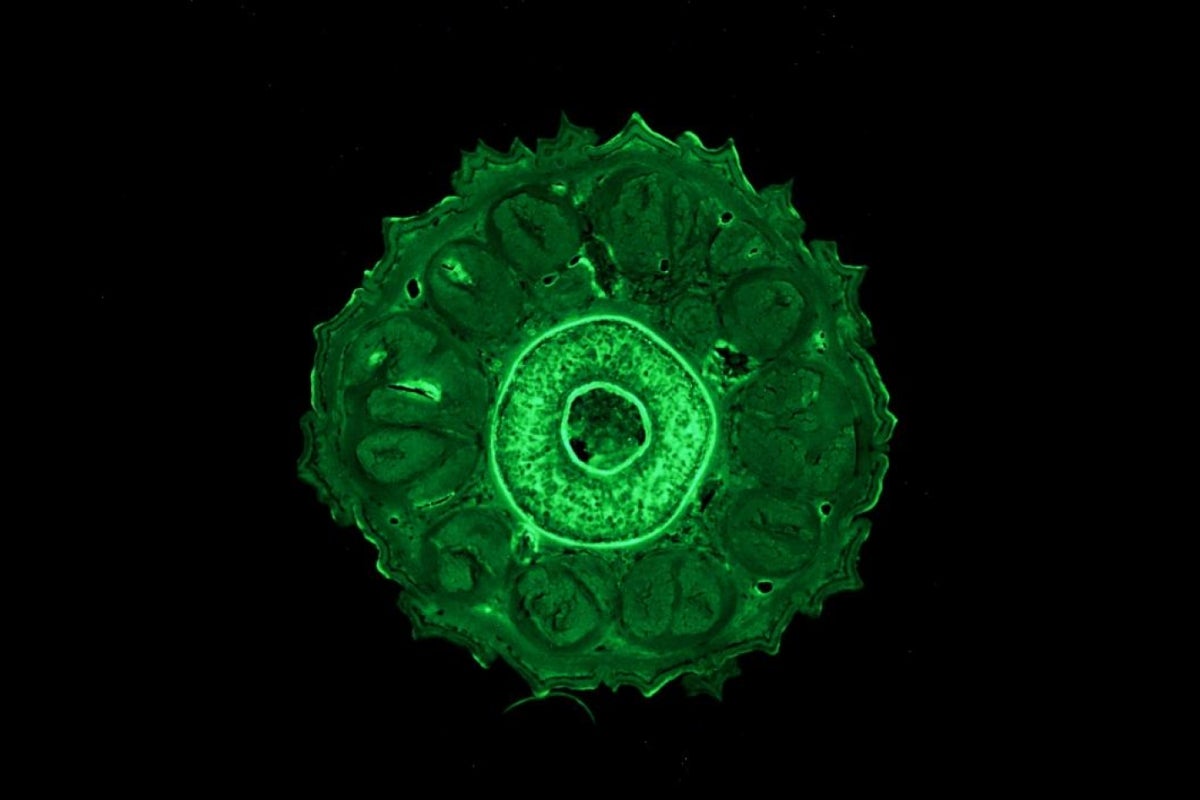
In one paper, scientists from ASU investigated the role of a muscle stem cell population called "satellite" cells. Regeneration involves making new muscle, cartilage and tendons and requires cells that will become these tissues in a regrown tail.
The researchers found that the muscle satellite cells in green anole lizards (Anolis carolinensis) do double duty and can become cartilage as well. This study provides the first functional description of this stem cell population in lizards.
“Satellite cells are a unique stem cell population that allows humans to grow and repair muscle tissue,” said senior author Jeanne Wilson-Rawls, associate professor with ASU School of Life Sciences. “Mammals, including mice and humans, have muscles that contain these cells. After an injury, these satellite cells can repair the remaining muscle, but they cannot replace lost muscle in humans, unlike in lizards.”
“Using cell culture techniques, we found that lizard satellite cells behave the same as mouse satellite cells,” said Joanna Palade, lead author of the first paper and graduate student in the ASU molecular and cellular biology graduate program. “However, while both cell types can differentiate into muscle, only lizard satellite cells can turn on the genes and proteins required to make cartilage.”
By studying the genetic programming in mice and lizards, the researchers hope to find the differences between them that make the lizard more capable of regeneration.
“Lizards and humans have most of the same genes,” said Kenro Kusumi, co-author of the study and professor with the school. “Working with expert computer scientists, we found the genes that control cartilage formation were turned on in lizard but not mouse satellite cells, pointing to the existence of a possible switch that must be activated for regenerative therapies.”

A green anole lizard (Anolis carolinensis) with a regenerated tail. Photo: Joel Robertson
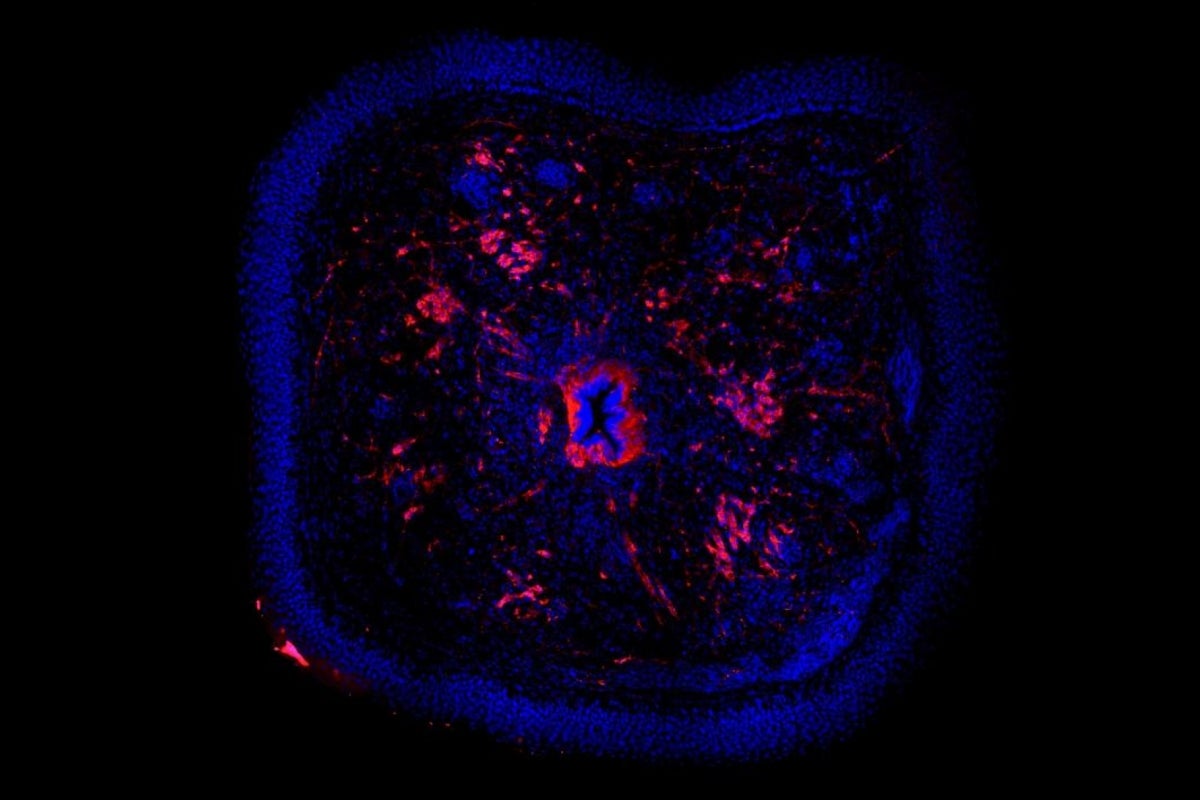
In a second study, the scientists found that nerve regeneration in particular, is a critical part of the tail regeneration process.
“Nerve regrowth is immediate in the regenerated lizard tail,” said Cindy Xu, co-author of the paper and also a graduate student in the program. “Regenerating nerves quickly repopulate the tail as muscle begins to form. As the neuromuscular junction matures, the nerves are pruned back but remain more numerous when compared to the original tail.”
In this study, the researchers allowed lizards to regenerate their tails up to 250 days and then studied the neuromuscular junctions — the connections between nerve and muscle — at different stages. Coordinated tail movements require effective communication between neurons and tail muscles through these neuromuscular junctions resulting in muscle contraction.
“Overall, we found that the regeneration of neuromuscular junctions in the lizard followed a pattern similar to development in mice and humans,” said Minami Tokuyama, co-author of the paper and former research technician in Kusumi’s lab. “However, the regenerated muscle ends up with a greater density of neuromuscular junctions, and studying these differences may be important in developing future therapies for humans.”
Together, these findings may bring researchers closer to solving the challenge of creating the capability for limb or organ regeneration in humans.
The research team included Kusumi, Palade, Tokuyama, Wilson-Rawls and Xu, as well as Jason Newbern and Alan Rawls, who are faculty with ASU’s School of Life Sciences; Rebecca Fisher with ASU School of Life Sciences and the University of Arizona College of Medicine-Phoenix; and Joshua Ho and Djordje Djordjevic from Victor Chang Cardiac Research Institute. The National Institutes of Health funded this research through grants.
More Science and technology

ASU to host 2 new 51 Pegasi b Fellows, cementing leadership in exoplanet research
Arizona State University continues its rapid rise in planetary astronomy, welcoming two new 51 Pegasi b Fellows to its exoplanet…

ASU students win big at homeland security design challenge
By Cynthia GerberArizona State University students took home five prizes — including two first-place victories — from this year’s…

Swarm science: Oral bacteria move in waves to spread and survive
Swarming behaviors appear everywhere in nature — from schools of fish darting in synchrony to locusts sweeping across landscapes…