Molded in clay: New antibacterials to kill germs

Microbiologist Shelley Haydel in her lab at the Biodesign Institute at Arizona State University. Photo by Ally Carr/ASU
For more than 20 years, microbiologist Shelley Haydel has been interested in antibacterial and antibiotic discovery. While preventing infections with vaccines is incredibly important, she prefers developing new ways to kill infectious bacteria.
“In a perfect world, we would have vaccines that prevent all human infections,” she said, “but we don’t live in a perfect world and we don’t have vaccines for all infections yet. In the meantime, we need to continue developing strategies to kill or inhibit the growth of the bacteria that are causing infections.”
Haydel and her collaborator, Dong-Kyun Seo, have received a $400,000 grant from the National Institutes of Health to test and refine a new antibacterial material, called artificial nanozeolites, over the next two years.
Sometimes in science the inspiration for a new medicine comes from an unexpected source. Just like ACE inhibitors that treat high blood pressure were derived from the pit viper and the active ingredient in the cancer-fighting drug Taxol was discovered in tree bark, the inspiration for Haydel’s new antimicrobial was found in the dirt.
Rub a little dirt on it
Years ago, Haydel, a microbiologist at ASU’s Biodesign Institute Center for Infectious Diseases and Vaccinology and the School of Life Sciences, began testing the antibacterial effects of natural clay minerals. Intrigued by the possibility of learning how natural clays were antimicrobial, Haydel tested dozens of different types of natural clay.
“We determined from the antibacterial natural clay that specific ions, iron, copper, cobalt, nickel and zinc, are released from the clay in water, and those ions are killing the bacteria in laboratory experiments,” said Haydel. “Additionally, we found that we can create antibacterial clays in the lab by adsorbing the ions to the clay.”
With a bit of help from science, Haydel in collaboration with Dong-Kyun Seo, a materials chemist in the School of Molecular Sciences, plan to test a new product that will mimic the clay’s antibacterial properties and protect against the dangers associated with harmful elements that are present in many clays.
“Basically we are taking the information that we learned from the clay research and are applying it to materials that are inexpensive and safe,” said Haydel.
Wonder material
“It goes back to my general philosophy,” said Seo (pictured left). “As a materials chemist, I really want to create a new material that can provide a direct impact to society as soon as possible. And I think we have that kind of material for antimicrobial applications. We have other applications too, like carbon dioxide separation, but it seems that antimicrobial applications will have the fastest impact.”
The secret to the success of this wonder material lies in the structure. Tiny holes inside zeolites, or pores, are just large enough to hold metal ions, like silver, that are important for killing bacteria. If you insert silver ions into the pores and then place the zeolites into water with bacteria, the ions will be released from the pores into the water and will kill the bacteria.
“The difference between a clay and zeolites is that a clay is a layered material,” said Seo, “Unlike zeolites, clay is inherently a nanomaterial made up of stacks of very thin layers. The antibacterial ions are sandwiched between the layers.”
Although clays and zeolites hold ions in different ways, they have a similar composition. Clays and zeolites are aluminosilicates, meaning they are a material made from Earth's most abundant elements: aluminum, silicon and oxygen.
“From her own perspective, Shelley was interested in the fact that our material is very pure,” said Seo, “It’s a synthetic material and there is better controllability, and better ways of optimizing the structure and properties. She felt this was a nice extension of her clay research.”
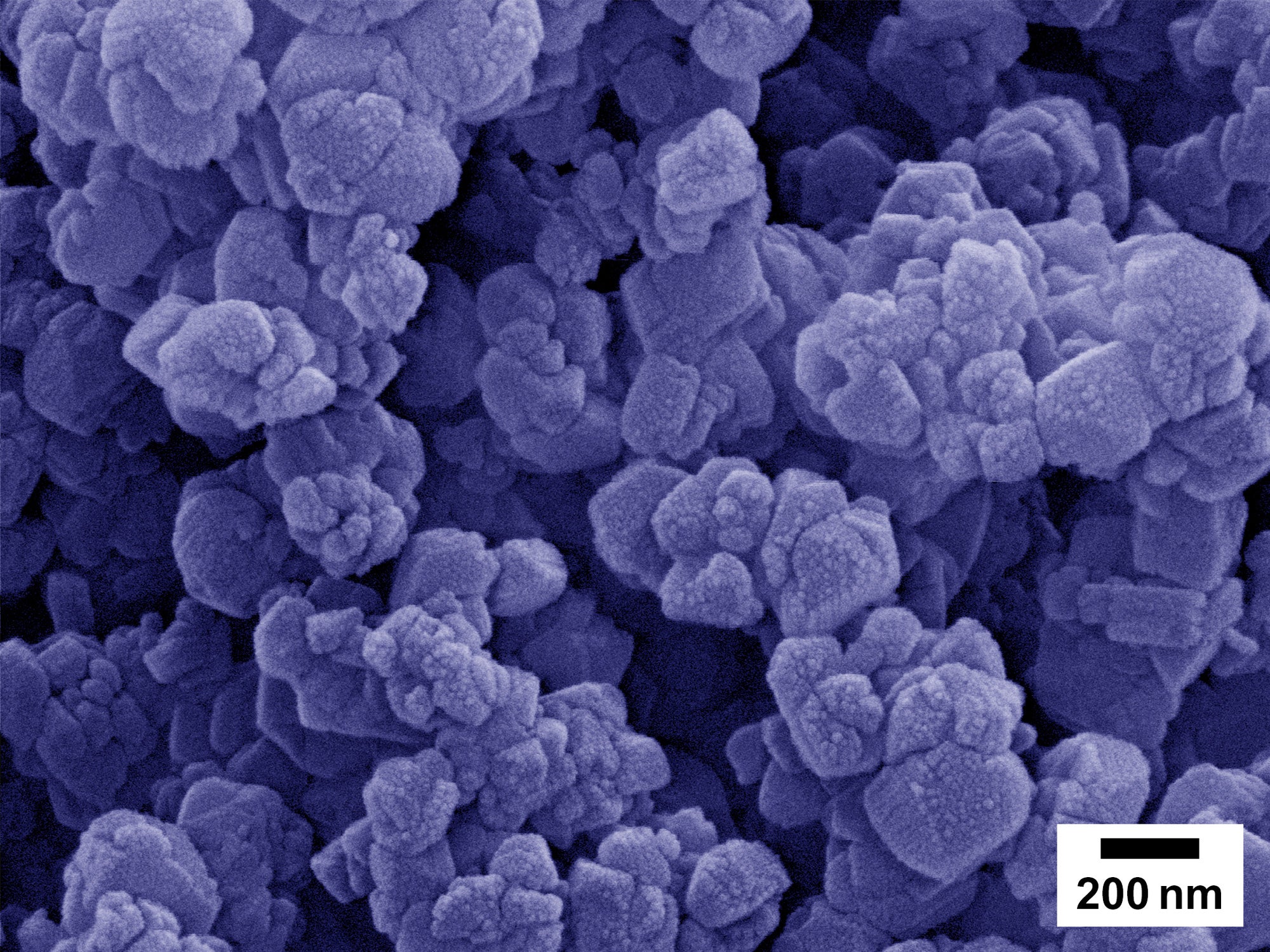
The new zeolites, or nano-structured zeolites, that Seo and Haydel will be testing are very tiny individual particles that combine into a larger particle, or aggregate particle. Think about how individual grapes connect to form a bunch of grapes. Each zeolite particle is very tiny (about 1/10,000th of a human hair) and thus silver ions can be released very effectively from the inside of the particle.
The 100 to 200 nanometer-size particles (aggregates) you see here are made of much smaller zeolite particles that are only around 20 nanometers.photo courtesy of Dong-Kyun Seo
Theoretically, the time for the ions to come out from a nanozeolite is 10,000 times shorter than what you expect from the micron-size zeolites that are commercially available now.
The faster the release of ions, the less ions are needed to make the antibacterial effective. Less ions means a cheaper product, since the bulk of the price is from silver. However, such tiny particles are difficult to handle and can even be harmful. If the nanoparticles are bunched into a much bigger size, they are much easier and safer to handle and use.
Living in a material world
“Once we develop products that are beneficial for whatever the infection is, let's use Buruli ulcer as an example, we should be able to make it from natural resources in any country,” said Haydel.
Buruli ulcer, a necrotic skin lesion caused by the bacterium Mycobacterium ulcerans, is the third most common infection in the world caused by mycobacteria, behind tuberculosis and leprosy.
“One of the interesting things about this particular disease is that the skin lesion is painless,” said Haydel. “Mycobacterium ulcerans produces a toxin that is analgesic, immunosuppressive, and destroys peripheral nerves.”
Haydel and Seo will be exploring the physical characteristics of their new nanozeolites to make a material that will adsorb damaging toxins such as these, which would further aid in wound healing.
“Buruli ulcer starts out as a small nodule, almost like a pimple, which does not hurt,” said Haydel.
Because it doesn’t hurt and seems unthreatening at first, people don’t tend to seek treatment until the wound becomes necrotic — the flesh starts to die.
According to the World Health Organization, 80 percent of those who begin treatment early can be cured, and those who wait have a 25 percent chance of becoming disabled.
A study in Cameroon calculated that, despite free treatment options for those affected by Buruli ulcer, the social and monetary cost was too high for patients to finish, or in some cases begin, treatment.
Four months was the average time spent in the hospital to treat Buruli ulcer, leading many family members to leave those who were receiving treatment because they needed to return home and work. Social isolation caused nearly 62 percent of patients to abandon treatment and return to their family.
“If we can develop better oral antibiotics for Buruli ulcer and develop something that can be applied topically,” she said, “then we will have complementary and effective treatments that are available in the communities, so that patients don’t have to go to the hospital.”
One of the goals is to create a highly effective, accessible and affordable treatment option for Buruli ulcer that won’t require those seeking treatment to leave their villages.
The long-term goal is for this material to be a complementary topical treatment for wound infections like Buruli ulcer or skin and soft-tissue infections causes by methicillin-resistant Staphylococcus aureus (MRSA), and used in conjunction with oral or injectable antibiotics.
“We plan to attack the infection from the outside, while oral or injectable antibiotics are attacking the bacteria on the inside,” Haydel said.
More Science and technology

ASU researcher part of team discovering ways to fight drug-resistant bacteria
A new study published in the Science Advances journal featuring Arizona State University researchers has found…

ASU student researchers get early, hands-on experience in engineering research
Using computer science to aid endangered species reintroduction, enhance software engineering education and improve semiconductor…

ASU professor honored with prestigious award for being a cybersecurity trailblazer
At first, he thought it was a drill.On Sept. 11, 2001, Gail-Joon Ahn sat in a conference room in Fort Meade, Maryland.…