ASU enters new $7M phase in program to develop radiation-absorption test

Biodesign interim executive director Josh LaBaer will lead a research team as part of a development project to produce a diagnostic test to measure an individual’s level of absorbed radiation in the event of an unplanned radiological or nuclear event.
Arizona State University’s Biodesign Institute announced today it is entering a new, $7 million phase of a multimillion-dollar, multi-institutional development project to produce a diagnostic test to measure an individual’s level of absorbed radiation in the event of an unplanned radiological or nuclear event.
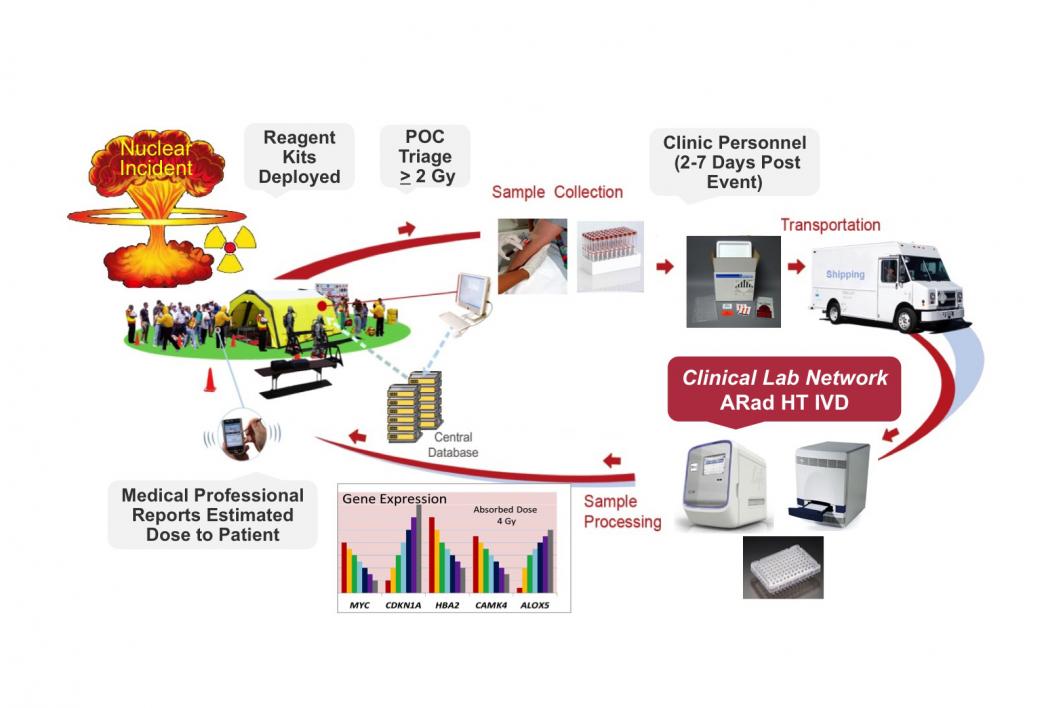
Currently, there is no U.S. Food and Drug Administration-cleared or -approved, high-throughput system to measure the radiation dose absorbed by individuals within a large population. The ASU Radiation Biodosimetry System (ARad), developed in collaboration with Thermo Fisher Scientific, is a real-time quantitative PCR-based (polymerase chain reaction), multi-gene panel designed to estimate absorbed radiation. The project is entering the verification phase with the goal of seeking clearance from the U.S. FDA.
“We are extremely proud to be moving forward into the device verification phase and planning validation studies needed for FDA submission. These efforts combine ASU’s development of radiation biomarkers, automation and software for estimating an individual’s absorbed dose with Thermo Fisher’s expertise in the product commercialization process,” said Joshua LaBaer, director of the Biodesign Institute’s Virginia G. Piper Center for Personalized Diagnostics. “Our research team’s ultimate goal is to translate our discoveries into an automated high-throughput diagnostic system for radiation biodosimetry that is capable of processing a high volume of blood samples per day.”
The project is part of a potential $34.7 million in total funds authorized by the Biomedical Advanced Research and Development Authority (BARDA), a federal agency within the Office of the Assistant Secretary for Preparedness and Response of the U.S. Department of Health and Human Services. The assay utilizes the Applied Biosystems brand of qPCR Dx instruments for gene expression analysis.
“Thermo Fisher and its long heritage of providing leading qPCR technologies for molecular detection is uniquely suited to drive the ARad Biodosimetry Project with ASU,” said Mark Smedley, president of Genetic Sciences at Thermo Fisher. “We are looking forward to continuing our partnership and enabling the next phase of this important work, which represents a novel approach to protect public safety.”
There is no FDA-approved test to rapidly screen a population for possible radiation exposure in the event of a nuclear incident. The project combine ASU’s development of radiation biomarkers, automation and software for estimating an individual’s absorbed dose with industry leader Thermo Fisher’s expertise in the product commercialization process.
“A critical part of the success of this project are the medical clinics and numerous volunteers that have provided blood samples to enable development of this medical device, many of whom are undergoing radiotherapy procedures,” said Kristin Gillis, senior scientific project manager of the ARad Radiation Biodosimetry Project.
Collaborators to the team include: Joshua LaBaer, director of the ASU Biodesign Institute and of the institute’s Virginia G. Piper Center for Personalized Diagnostics; Sally Amundson, professor of radiation oncology at Columbia’s Center for Radiological Research; and principal investigators at City of Hope, Stanford University and Mayo Clinic, providing critical access to patient clinical samples.
This project has been funded in whole or in part with federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under Contract No. HHS0100201000008C.
More Science and technology

ASU water polo player defends the goal — and our data
Marie Rudasics is the last line of defense.Six players advance across the pool with a single objective in mind: making sure that…

Diagnosing data corruption
You are in your doctor’s office for your annual physical and you notice the change. This year, your doctor no longer has your…
Large-scale study reveals true impact of ASU VR lab on science education
Students at Arizona State University love the Dreamscape Learn virtual reality biology experiences, and the intense engagement it…