Winner-take-all synthetic gene circuit opens new pathways to disease treatment

A new process for inserting synthetic gene circuits into host cells, developed by a team of bioengineers at Arizona State University, has broad implications for improving the effectiveness of a range of disease therapies.
Synthetic biology is an interdisciplinary research field that uses engineering principles to create biological components that don’t exist in the natural world. These synthetic components mimic naturally evolved organisms, but are customized to fight disease, including cancer.
A paper recently published in Nature Communications, “Winner-Takes-All Resource Competition Redirects Cascading Cell Fate Transitions,” outlines how gene circuits can be reconfigured so that they do not overwhelm the host cells.
“We connect circuits together like a Lego chain and insert them into a host cell,” explained lead author Xiaojun Tian, an assistant professor in the School of Biological and Health Systems Engineering at ASU. “The circuits in the chain are designed to perform different functions, but they must compete with each other for the cell’s limited resources.”
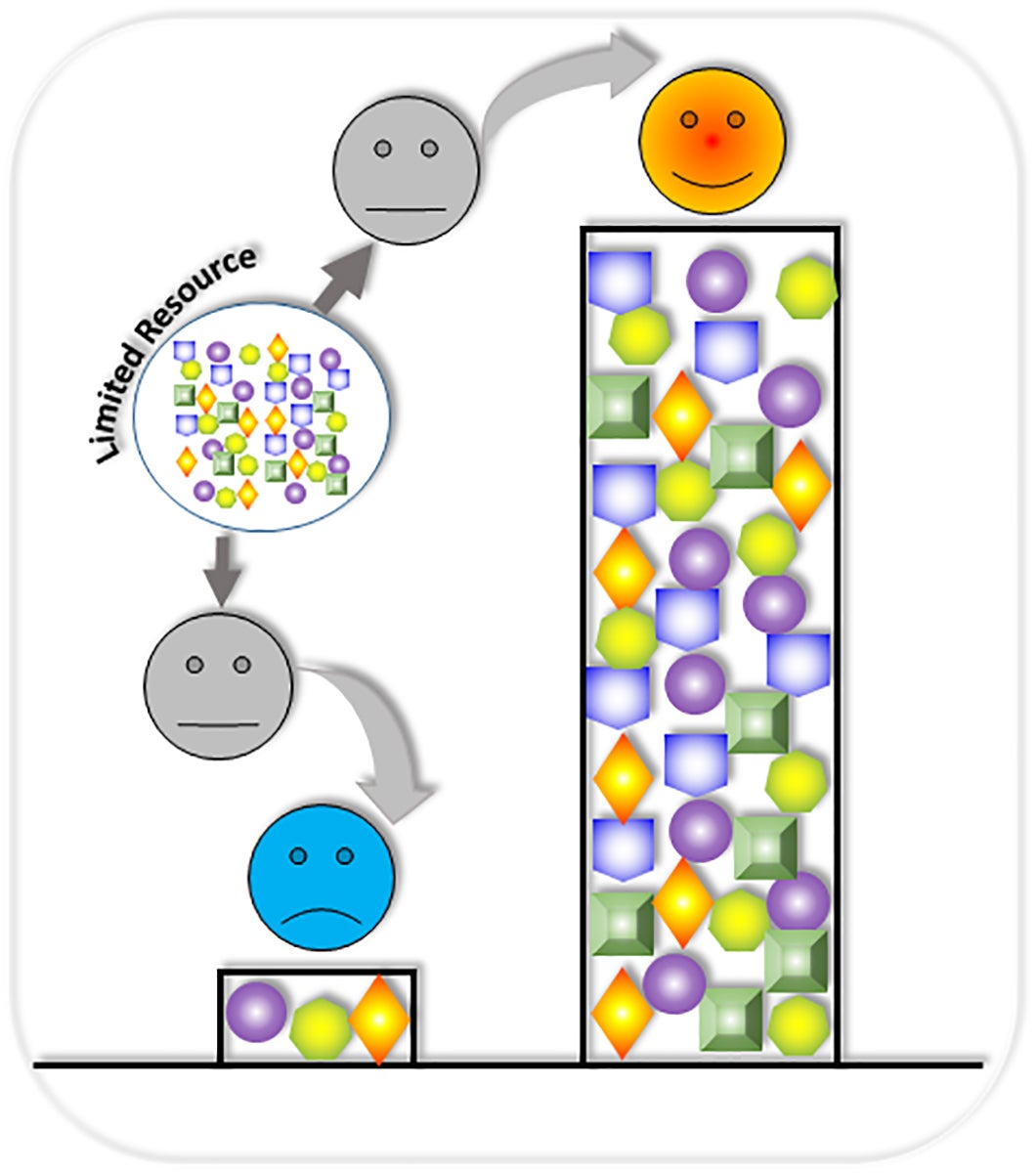
Assistant Professor Xiaojun Tian’s research reveals a novel winner-take-all resource competition between synthetic gene circuits within one host cell. Graphic created by Xiaojun Tian/ASU
Competition for resources has been a challenge in the synthetic biology field since its inception 20 years ago.
“We would find circumstances where one gene circuit in a chain would consume 90% of a host cell’s available resources, leaving only 10% for the remaining circuit,” Tian said.
Tian’s team devised a way to insert individual gene circuits into multiple host cells that work collectively. Each cell performs a specific function, eliminating the undesired competition for resources of any host cell.
“Instead of dividing resources, each cell can perform 100% of its assigned workload,” said Tian. “The host cells perform as a connected unit without depleting any one cell’s resources — and each gene circuit becomes a winner.”
The technology has broad implications for cancer treatment, with future applications for other diseases on the horizon. Ninety percent of cancer deaths are due to metastasis — the spread of cancer cells to other sites in the body. However, treatment resistance is still a major problem in cancer therapeutics.
“There are many different kinds of cells in a cancer mass,” said Tian. “Some cells are responsive to chemotherapy and others are not, causing treatment resistance."
New multitasking synthetic gene circuitry configuration can be constructed to prevent the cells from metastasizing in the first place, while simultaneously making them more receptive to treatment.
Tian explains that multicellular synthetic circuits will be a much more effective way to treat cancer.
The research team also includes Rong Zhang, Hanah Goetz, Juan Melendez-Alvarez, Jiao Li and Xiao Wang from ASU and Tian Ding from Zhejiang University in Hangzhou, Zhejiang, China. The research was funded by the National Science Foundation and the ASU Dean’s Fellowship.
Top photo by Gladson Xavier from Pexels.
More Science and technology

ASU and Deca Technologies selected to lead $100M SHIELD USA project to strengthen U.S. semiconductor packaging capabilities
The National Institute of Standards and Technology — part of the U.S. Department of Commerce — announced today that it plans to award as much as $100 million to Arizona State University and Deca…

From food crops to cancer clinics: Lessons in extermination resistance
Just as crop-devouring insects evolve to resist pesticides, cancer cells can increase their lethality by developing resistance to treatment. In fact, most deaths from cancer are caused by the…

ASU professor wins NIH Director’s New Innovator Award for research linking gene function to brain structure
Life experiences alter us in many ways, including how we act and our mental and physical health. What we go through can even change how our genes work, how the instructions coded into our DNA are…