Hydrocephalus is among the neurological disorders for which modern medical science remains stymied in the search for a cure. Even treatment for what is commonly called “water on the brain” is looked upon as antiquated in an age of expanding technological innovation.
Characterized by an abnormal buildup of cerebrospinal fluid in the brain, untreated hydrocephalus can trigger swelling, headaches and convulsions, impair cognitive functions and walking and even cause death.
The condition affects people of all ages, including infants — an average of one of every 500 newborns in the United States alone — as well as older children and adults, who can develop hydrocephalus from many conditions. It also affects people with post-traumatic stress disorder, or PTSD, many of whom are current or retired members of the military.
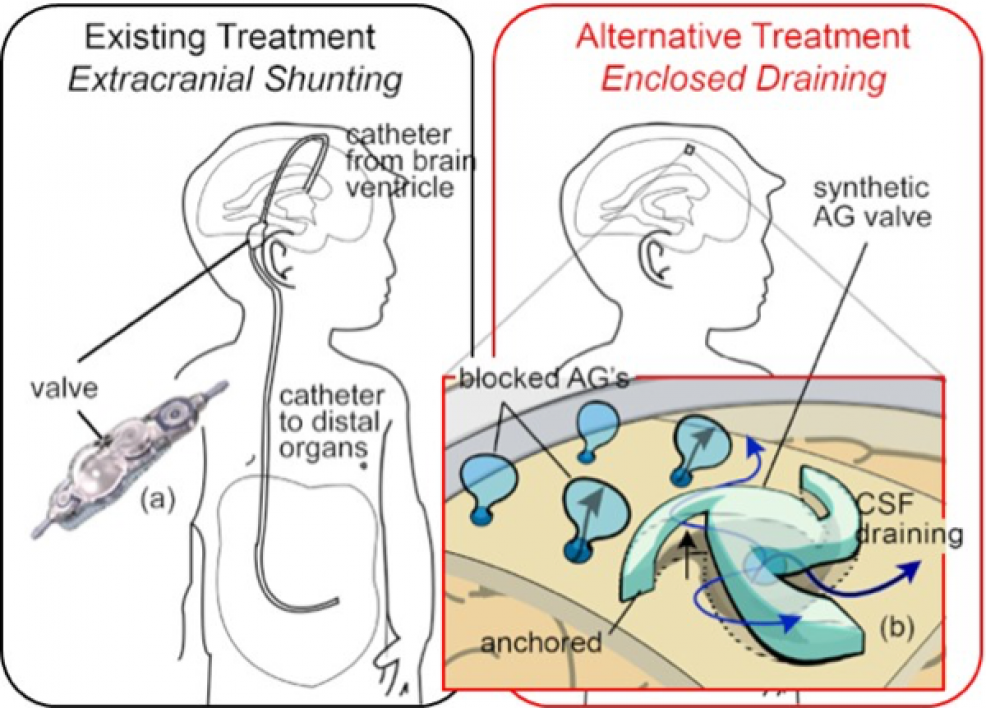
The current treatment process, called shunting, involves diverting the excess fluid from the brain using catheters. The tubing is routed from inside the skull and then underneath the skin to another cavity in the body, such as the abdominal cavity or the heart, where it is absorbed back into the body.
Shunting, however, has a failure rate of about 40% within two years after the procedure is performed and more than 95% within a decade.
But hope for a considerably less invasive and more resilient treatment may lie in advances in one especially fast-growing sphere of technology: micro-electro-mechanical systems, called MEMS.
These miniaturized electrical and mechanical devices are used mostly in semiconductor manufacturing, cell phones, digital displays, wireless communications, aerospace and automotive technologies, but their capabilities are being more fully explored beyond those applications.
Junseok Chae, a professor of electrical engineering in the Ira A. Fulton Schools of Engineering at Arizona State University, has been honing his expertise in the use of MEMS technology to develop medical devices.
In his research laboratory at Arizona State University’s Tempe campus, Professor Junseok Chae talks about progress he has been making through a collaboration with neurosurgical experts at the Barrow Neurological Institute to develop a more effective and less invasive treatment for hydrocephalus. Photo by Connor McKee/ASU
Progress on that endeavor recently helped earn two grants totaling $2 million from the U.S. Army’s Congressionally Directed Medical Research Programs. The funding supports an ongoing collaboration to develop brain implants that treat hydrocephalus. The work involves Chae and neurosurgical experts at Barrow Neurological Institute in Phoenix, one of the premier neurosurgery centers in the United States, and at Phoenix Children’s Hospital.
The partnership began in 2006, when Dr. Ruth Bristol, a pediatric neurosurgeon at Phoenix Children’s Hospital who has been treating patients with hydrocephalus for almost 20 years, asked Chae to work on a more effective treatment. That request has led to more than 12 years of multidisciplinary collaboration that also involves Dr. Mark Preul, a neurosurgeon and director of neurosurgery research at the Barrow Neurological Institute. Bristol is also with the institute.
Preul’s expertise includes the development and testing of advanced technologies for neurosurgery. One of the Army medical research program’s grants went to ASU to support Chae’s efforts. The other grant went to the Barrow Neurological Institute to fund work led by Preul at Barrow’s neurosurgery laboratory.
“We want to implant a device manufactured from MEMS technology that will drain the cerebral spinal fluid just like the way the body does it naturally when there is no hydrocephalus,” said Chae, who teaches in the School of Electrical, Computer and Energy Engineering, one of the six Fulton Schools.
Cerebrospinal fluid, which is a watery fluid produced by special cells in the brain, surrounds and bathes the brain and the spinal cord. Almost a pint of it is produced every day in the brain’s cavities, called ventricles.
The fluid flows around the brain and spinal cord through the spaces between the brain’s membranes before it is absorbed into the bloodstream. Along the way, cerebrospinal fluid performs critical functions such as protecting the brain by acting as a shock absorber, bringing nutrients to the brain and taking waste out.
In a healthy brain, Chae explained, the fluid circulates through the brain and then flows through tissue structures called arachnoid granulations — projections of the arachnoid membrane — which rest on one of the brain’s meningeal layers that support the framework for cerebral and cranial blood vessels.
“What we want to do is implant our MEMS device onto the meningeal layer to act like natural arachnoid granulation,” Chae said.
The arachnoid granulations regulate the flow of cerebrospinal fluid from the subarachnoid space to the sinus space where it is absorbed back into the body’s blood stream.
In cases of hydrocephalus, the flow, drainage and absorption process gets blocked, causing the troublesome cerebrospinal fluid buildup in the brain.
Chae’s team describes its brain implant as an artificial arachnoid granulation that will “emulate the body’s arachnoid granulation valve system and restore brain fluid flow to its natural pathway by mimicking the body’s natural process,” he said.
To do this, the researchers are using MEMS technology to manufacture the tiny device that regulates the fluid flow through the properties of a hydrogel made of a biocompatible polymer, which has a unique swelling feature enabling it to regulate the flow of cerebrospinal fluid between the subarachnoid and sinus spaces.
Electrical engineering doctoral student Seunghyun Lee examines one of the small implantable devices designed to help prevent the debilitating effects of hydrocephalus. Photo by Connor McKee/ASU
“This will be a hydrocephalus treatment that is a minimally invasive procedure compared to the current bulky shunt treatment system. It won’t require any catheters, which are currently the biggest cause of the failure of the shunt system,” Chae said.
The new treatment “will have no implanted batteries or electronics but will operate with a fully passive artificial valve to mimic the body’s natural regulation of brain fluid,” Chae said. “The fluid will flow into an outer layer of the brain (the superior sagittal sinus), where it can be reabsorbed into the blood stream.”
Chae, Bristol and Preul are among the co-authors of three articles published in research journals that report on recent technical advances related to the new treatment — in IEEE Transactions on Biomedical Engineering; the Annals of Biomedical Engineering, part of the Springer Nature Journal, and in the current edition of the American Chemical Society’s ACS Sensors journal.
Despite the promise of the new treatment, Chae cautions against expectations for it to be put into practice in the near future.
“Good things are happening in our research, but this is still a long-term exploratory project,” he said. “We have to go through the Food and Drug Administration’s process and many other validations before we are certain we can could deploy this treatment in a completely safe manner.”
Success in the endeavor, however, would “translate into dramatically improved quality of life for hydrocephalus patients,” Chae said.
Chae credits some of the progress on the project to his student lab assistants.
Electrical engineering doctoral student Seunghyun Lee has contributed to development of a MEMS device, including testing it in the lab, collecting testing data and analyzing the data using advanced statistical methods.
Daniel Beltran, an electrical engineering undergraduate student, has been helping to manufacture the MEMS device, as well as develop the lab setup to test multiple devices simultaneously.
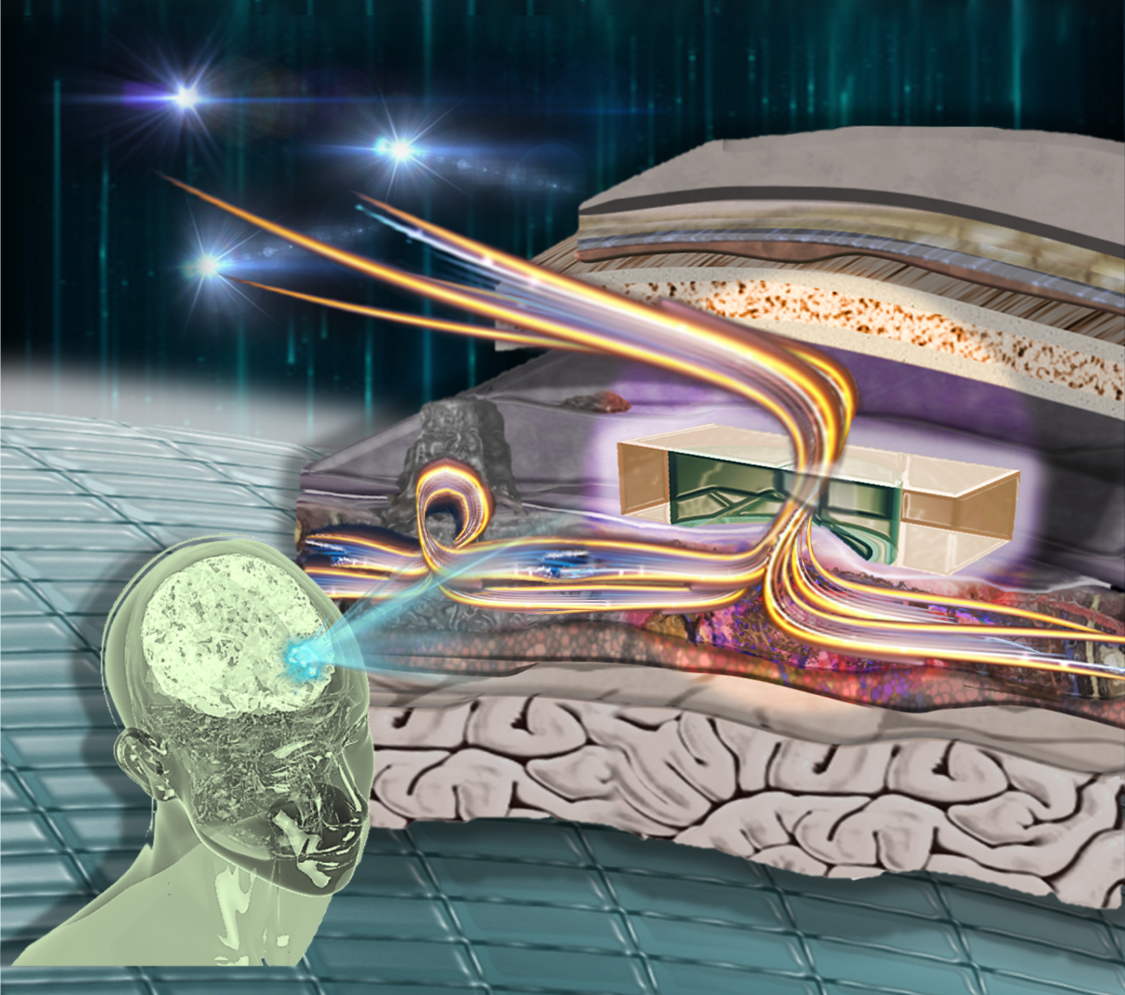
Top photo: Researchers are using advanced micro-electro-mechanical systems, or MEMS, technology to manufacture small implants that will restore the normal flow of cerebrospinal fluid through the brain to relieve pressure resulting from hydrocephalus. Photo by Connor McKee/ASUMore Science and technology

Lucy's lasting legacy: Donald Johanson reflects on the discovery of a lifetime
Fifty years ago, in the dusty hills of Hadar, Ethiopia, a young paleoanthropologist, Donald Johanson, discovered what would become one of the most famous fossil skeletons of our lifetime — the 3.2…

ASU and Deca Technologies selected to lead $100M SHIELD USA project to strengthen U.S. semiconductor packaging capabilities
The National Institute of Standards and Technology — part of the U.S. Department of Commerce — announced today that it plans to award as much as $100 million to Arizona State University and Deca…
From food crops to cancer clinics: Lessons in extermination resistance
Just as crop-devouring insects evolve to resist pesticides, cancer cells can increase their lethality by developing resistance to treatment. In fact, most deaths from cancer are caused by the…