ASU research group uncovers a greener alternative in sustainable chemistry

From left: Thao Nguyen, Ryan Trovitch, Anuja Sharma, A K M Fazlul Karim Rasel, Matthew Mena and Christopher Rock.
Catalysis is an essential component of industrial chemistry and the economic and societal benefits of catalysis are almost incalculable.
Catalysts increase the speed and selectivity of chemical reactions by lowering the energy barriers to the critical bond-breaking and -making processes. Over 80% of all industrial chemical processes involve catalysis and roughly 30% of the gross domestic product of many industrialized nations depends upon catalysis. It's crucial to the production of the fertilizers that are used for crop production across the globe. It has been estimated that over half of the population of the world would not be alive today if it were not for the invention of the catalytic production of the essential feedstock for fertilizer production, ammonia.
Precious metal compounds are regularly used to catalyze industrial chemical reactions; however, these metals can also exhibit toxicity and be expensive to work with, and the world's supply of many is being rapidly depleted. Fortunately, green chemistry principles can be applied to develop more sustainable and environmentally-responsible replacements for these precious metals that use more benign and Earth-abundant base metals.
Ryan Trovitch's group in the School of Molecular Sciences at Arizona State University is leading the way in the development of new cost-effective and environmentally friendly catalysts for chemical reactions based on these principles. The Trovitch Group is showing that underutilized metals such as cobalt and vanadium can be used to catalyze a variety of important chemical reactions under mild conditions. A central scientific theme in the new catalytic processes invented by the Trovitch group is that the part of the molecular structure of the catalyst that surrounds the metal (the ligands) simultaneously stabilizes the metal center and promotes the fundamental bond-breaking and -making required to initiate chemical reaction.
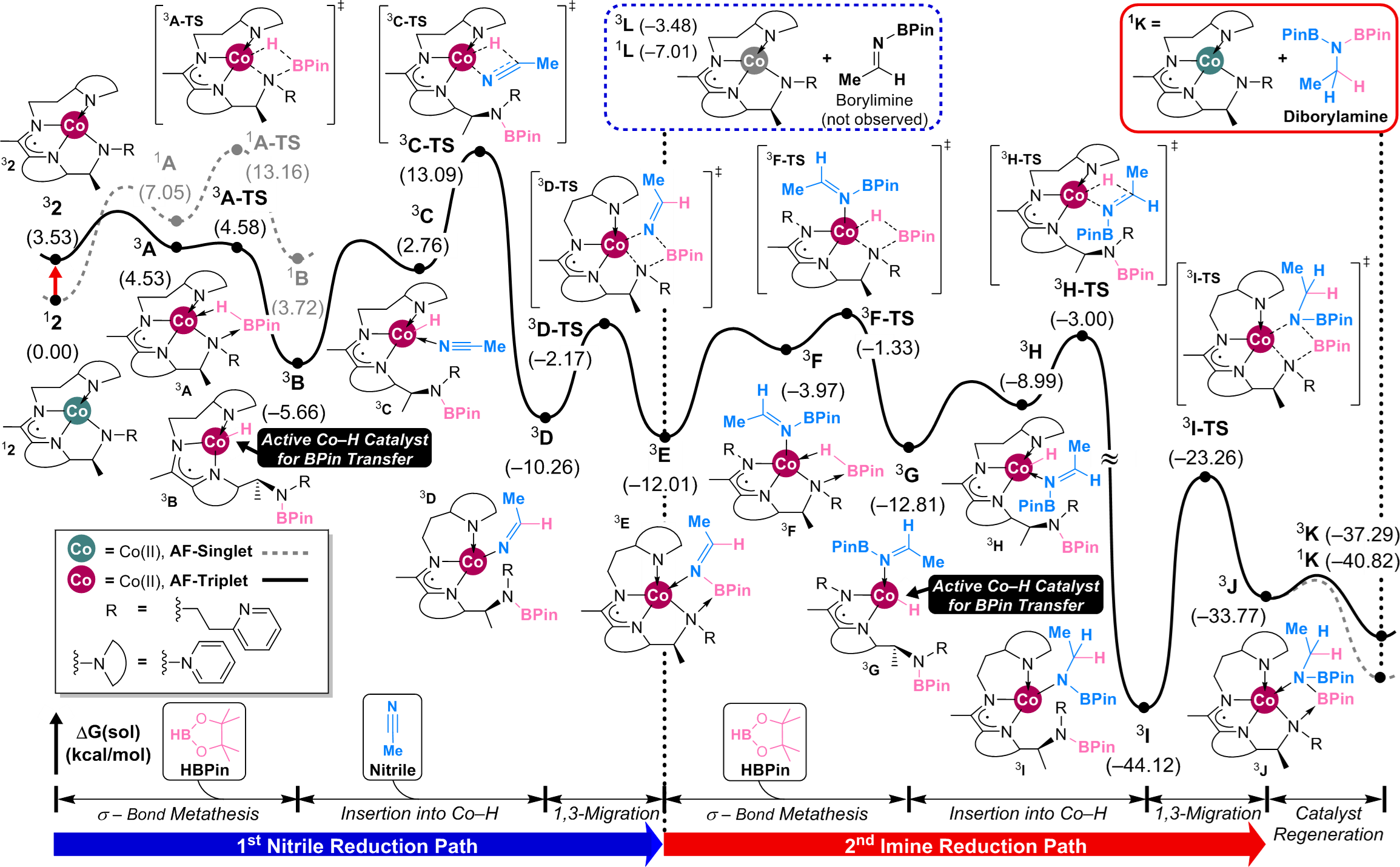
Their research is described in two papers recently published in the Journal of the American Chemical Society: "Redox-Noninnocent Ligand-Supported Vanadium Catalysts for the Chemoselective Reduction of C═X (X = O, N) Functionalities" and "Efficient Cobalt Catalyst for Ambient-Temperature Nitrile Dihydroboration, the Elucidation of a Chelate-Assisted Borylation Mechanism, and a New Synthetic Route to Amides."
Efficient cobalt catalyzed nitrile hydroboration has been achieved by using a ligand that simultaneously allows for a spin transition, redox non-innocence and inner-sphere boryl group transfer.
“Taken together, these publications highlight how ligand-based reactivity can be utilized to design next-generation base metal catalysts,” explained Trovitch, an associate professor in the School of Molecular Sciences.
Compared to the precious metals normally used in catalysts, cobalt and vanadium are relatively abundant in the Earth's crust. Cobalt ore deposits are found in Democratic Republic of Congo, Canada, Australia, Zambia and Brazil and vanadium ore is mined in South Africa, Russia and China.
Using cobalt and vanadium metals as their basis, the Trovitch group has discovered new catalysts for efficient synthetic routes to alcohols, amines and amides, all basic chemical structures important in a wide range of chemical manufacturing and processing. In addition to describing new reactions, their recent publications describe the fundamental mechanistic principles that allow these new catalysts to function. Characterization of the new catalyst materials relied on ASU’s single crystal X-ray diffraction, electron paramagnetic resonance and nuclear magnetic resonance facilities.
Ian Gould, associate director of the School of Molecular Sciences, said that "the detailed and careful mechanistic detail described in these studies is likely to influence and inform future approaches to the development of new base metal catalysis."
More Science and technology

ASU-led space telescope is ready to fly
The Star Planet Activity Research CubeSat, or SPARCS, a small space telescope that will monitor the flares and sunspot activity…

ASU at the heart of the state's revitalized microelectronics industry
A stronger local economy, more reliable technology, and a future where our computers and devices do the impossible: that’s the…

Breakthrough copper alloy achieves unprecedented high-temperature performance
A team of researchers from Arizona State University, the U.S. Army Research Laboratory, Lehigh University and Louisiana State…