On Nov. 25, 1901, a 51-year-old woman is admitted to a hospital in Frankfurt, Germany, displaying a bizarre constellation of symptoms. Her behavior is erratic. She shows signs of paranoia as well as auditory hallucinations, disorientation and severe memory impairment. Asked to write her own name, she manages “Mrs.,” then lingers over the page, unable to remember the rest. “I have lost myself,” she tells the attending physician.
Over time, she will withdraw into her own inscrutable universe, before dying on April 9, 1906.
The tragic case of Auguste Deter might have vanished into the recesses of medical history, but for the following fact: Her doctor, Alois Alzheimer, made a thorough examination of her medical condition, including her excised brain, discovering the telltale amyloid plaques and neurofibrillary tangles characteristic of her illness. Auguste Deter was the first person diagnosed with Alzheimer’s disease.
Today, society faces an epidemic of Alzheimer’s, with some 5 million afflicted in the U.S. alone. The number is projected to swell to 14 million by midcentury, according to the Centers for Disease Control and Prevention. Of the top 10 leading fatal illnesses, Alzheimer’s remains the only one that cannot be prevented, treated or cured.
In new research appearing in the journal Alzheimer’s and Dementia, Diego Mastroeni, Paul Coleman and their colleagues at the ASU-Banner Neurodegenerative Disease Research Center (NDRC) and the Biodesign Center for Bioenergetics investigate the role of mitochondria in Alzheimer’s disease pathology. Mitochondria act as energy centers for cells and are of central importance in health and disease.
The study builds on earlier work suggesting gene mutations affecting mitochondrial function may be critical in the development — and pitiless progression — of the disease.
Diego Mastroeni is an assistant research professor in the ASU-Banner neurodegenerative Disease Research Center.
“Age-related neurodegenerative diseases, like Alzheimer’s, progress over a long period of time before they become clinically apparent. The earliest physiological and molecular events are largely unknown,” said Mastroeni. “Findings from our laboratory have uncovered early expression changes in nuclear-encoded, but not mitochondrial-encoded mRNAs occurring in one’s early 30s, giving us a glimpse into what we suspect are some of the earliest cellular changes in the progression of Alzheimer’s disease.”
Results of the new study show that specific classes of genes associated with mitochondrial cell respiration display reduced expression levels in patients with Alzheimer’s disease, compared with normal patients.
The study also examines gene expression in subjects whose brains show an intermediate level of illness known as mild cognitive impairment. Here, the opposite effect is observed, with relevant genes exhibiting increased levels of expression. The authors suggest this observation may point to some kind of compensatory mechanism in the brain attempting to stave off the disease in its earlier stages.
Further, the study proposes that restoring a specific set of damaged genes linked to mitochondrial function and located in the nuclear DNA of cells may offer a promising strategy for halting the disease’s advance.
Assault on identity
Alzheimer’s — the most common form of dementia — is a progressive, degenerative disease of the brain. While commonly associated with elderly individuals, this devastating illness is now believed to have its origins much earlier, infiltrating the nervous system decades before the onset of clinical symptoms. Indeed, the greatest obstacle to successful treatment of Alzheimer’s is the fact that the disease is typically not recognized until its progress has irreparably ravaged the brain.
The disease often begins with mild memory loss, which may interfere with normal conversation. While advancing age remains the leading risk factor for Alzheimer’s, some individuals are also genetically predisposed. Other risk factors include high cholesterol, heart disease, stroke and high blood pressure. Today, Alzheimer’s is the fifth-leading cause of death in adults 65-85 years old.
Paul Coleman is a research professor in the ASU-Banner Neurodegenerative Disease Research Center.
Despite the increasingly pronounced effects of dementia, a definitive diagnosis of Alzheimer’s disease usually requires the post-mortem examination of brain tissue and identification of two stereotypic symptoms, known as plaques and tangles. More recently, new imaging technology has enabled researchers to detect these symptoms in living brains, though Coleman is cautious about their interpretation:
“Although plaques and tangles remain as the definitive neuropathological hallmark of the disease, plaques do not correlate at all with degree of cognitive impairment in [Alzheimer’s] and tangles correlate only slightly,” he said. “We further know that plaques and tangles are late comers in the cascade of events that cause the dementia of [Alzheimer’s].”
Alzheimer’s is believed to account for 60-70 percent of dementia cases. As the disease progresses, symptoms become more severe, including erosion of language ability, physical disorientation and behavioral transformations, often involving the withdrawal from family and society. Over time, bodily functions are lost, ultimately leading to death. Life expectancy for Alzheimer’s patients varies, but three to nine years following diagnosis is typical.
Quick energy
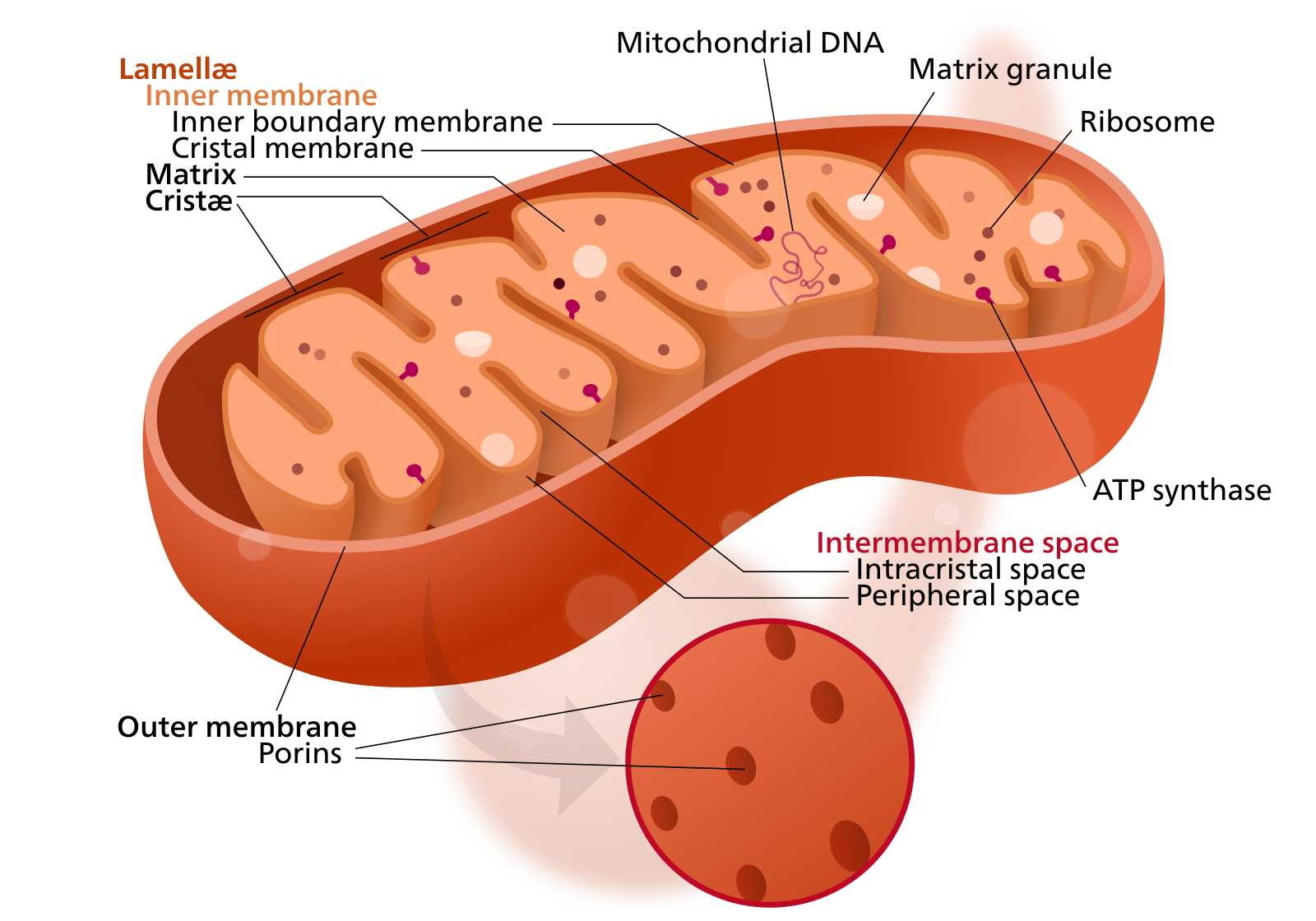
Mitochondria — membrane-bound organelles found in all eukaryotic organisms — are often called the powerhouses of the cell. Through a process known as oxidative phosphorylation, they produce most of the cell’s chemical energy in the form of adenosine triphosphate or ATP.
In addition to supplying cellular energy, mitochondria are involved in cell signaling, cellular differentiation and cell death, as well as in cellular growth and the maintenance of the cell cycle.
Because mitochondria play such an important role in the cell, mitochondrial dysfunction has been implicated in a broad range of illness, including cardiovascular disease, autism, schizophrenia, bipolar disorder, epilepsy, stroke, Lou Gehrig’s disease and diabetes along with forms of dementia including Alzheimer’s.
Unsurprisingly, defects in mitochondrial function more severely affect energy-hungry organ systems in the body, particularly muscles, the GI tract and the brain — an organ making up just 2 percent of a person’s weight while consuming 20 percent of the body’s total energy budget.
Mitochondria are membrane-bound organelles present in eukaryotic cells. Their essential role is to supply cells with energy in the form of ATP. Mitochondrial dysfunction is implicated in a range of diseases, including Alzheimer's.
Mitochondria are unique among the cell’s organelles, as they possess their own DNA, distinct from the DNA contained within the cell’s nucleus. This strange state of affairs is due to mitochondrial evolution. Mitochondria are descended from free-living bacteria that colonized other cells some 2 billion years ago. After being incorporated into nucleated cells, these endosymbionts, as they are known, lost much of their original machinery, yet retained their own complement of DNA.
In addition to the role of mitochondrial dysfunction in disease, the gradual degradation of mitochondrial integrity is believed to play a central role in the normal process of aging.
Broken genes
The current study examines tissue from the hippocampus, a structure critical for memory and one severely impacted by the advance of Alzheimer’s. Using microarray technology, the authors examined hippocampal tissue from an aging cohort-44 normal brains from 29-99 years of age, 10 with mild cognitive impairment and 18 with Alzheimer’s disease.
Gene expression was examined for two sets of genes, 1 encoding mitochondrial DNA and the other, in the nuclear DNA. The two sets of genes both coded for proteins associated with a mitochondrial complex essential for oxidative phosphorylation (OXPHOS), producing energy in the form of ATP for the cell.
Intriguingly, while the mitochondrial genes themselves were largely unaffected, the nuclear genes associated with the OXPHOS complex underwent significant modification, depending on the tissues examined. The microarray data revealed substantial down-regulation of nuclear-encoded OXPHOS genes in Alzheimer’s tissue, a finding also found in normally aging brains.
The same genes, however, were up-regulated in the case of mild cognitive impairment, a precursor to Alzheimer’s disease. The authors suggest this effect may be due to a compensatory mechanism in the brain in response to early pathology.
The findings are consistent with earlier work establishing that accumulations of amyloid beta (Aβ) in neurons, a hallmark of Alzheimer’s, are directly implicated in mitochondrial dysfunction. The pronounced effect on nuclear-encoded but not mitochondrial-encoded OXPHOS genes may point to dysfunctions in the transport of molecules from the cell nucleus to the mitochondria.
“Our work on mitochondria offers the promise of a reliable marker appearing earlier in the course of the disease — one which more closely correlates with the degree of dementia than the current diagnostic of plaques and tangles," Coleman said.
Precise mechanisms of mitochondrial decline in aging and Alzheimer’s have yet to be teased out and will be the focus of continuing research. The study suggests that therapies aimed at restoring function in nuclear-encoded OXPHOS genes may provide an exciting new avenue for treatment of Alzheimer’s.
More Science and technology

The Polytechnic School at ASU hits milestone mark at 10 years
The year was 2014.Taylor Swift released “1989.” "Frozen” and its soundtrack were stuck in the minds of many.Facebook was still the most popular social media site, and “Happy” by Pharrell…

Study finds cerebellum plays role in cognition — and it's different for males and females
Research has shown there can be sex differences for how male and female brains are wired.For example, links have been made between neurobehavioral diseases — such as attention-deficit/hyperactivity…

Artificial intelligence drives need for real data storage innovations
In southeastern Mesa, Arizona, construction crews are hard at work on a state-of-the-art data center. The $1 billion facility will open in 2026 and provide approximately 2.5 million square feet…