The hottest technology in bioscience will soon bring a new coolness factor to world-class Arizona State University research.
The coolest new way to take a near-atomic resolution snapshot of life at work is cryo-Electron Microscopy (cryo-EM) — lauded recently by Nature as its 2015 "Method of the Year" — and now coming soon to ASU.
“ASU has been a national leader in atomic-resolution electron microscopy since the 1970s and remains at the cutting edge today, expanding the boundaries of our knowledge in areas such as structural biology,” said Sethuraman “Panch” Panchanathan, chief research and innovation officer and executive vice president at ASU. “The National Science Foundation’s decision to add cryo-EM, and hence biology, to the world-leading electron microscopy facilities at ASU is a testament to both our world-class expertise and our emerging and breakthrough innovations.”
Life at work
Recent advancements in electron microscopes and the development of cryo-EM have made it possible for researchers to see the vital details — down to the molecules of important building blocks of life for the birth, life and death of cells, and ultimately, our health.
Petra Fromme, ASU professor in the School of Molecular Sciences and director of the Biodesign Institute’s Center for Applied Structural Discovery, will be using cryo-EM to peer into tough to solve membrane proteins and determine how they work.
Sandwiched within the membrane of living cells membrane proteins can act as a doorway for drug delivery, facilitate viral and bacterial infection and defense, and regulate cell growth, and yet relatively little is known about these important membrane proteins because, until now, they have been particularly difficult to solve.
Membrane proteins are important building blocks of life that catalyze key processes in biology including conversion of sunlight into chemical energy in the process of photosynthesis, and they also are of extreme importance for human health with 40 percent of all current drugs targeted to membrane proteins. While more than 100,000 structures of proteins have been determined to date, less than 600 membrane protein structures have been unraveled.
The novel cryo-EM technology will ideally complement the breakthrough new discoveries by the ASU interdisciplinary team.
“While our new X-ray technology with free electron lasers will allow us to determine real-time movies of biomolecules at work under natural room temperature conditions at atomic resolution using nanocrystals, cryo-EM will allow us to determine structures of large complexes based on frozen samples down to the level of molecules,” said Fromme.
Fromme is very enthusiastic about the potential and applications of cryo-EM at ASU: “The new cryo-EM technology will allow us to unravel structures of huge complexes that can not be crystallized. My group is very excited to study the structure and function of supercomplexes of photosystems with their huge antenna systems in photosynthesis and investigate the interaction of viruses with human cells at the molecular level.”
A brief history of EM
Corrective vision
Electron microscopes have been around for 80 years for the study of materials, but could not peer at the atomic level because of the imperfections of the electron lenses. Recent advances have paved the way for more advanced instruments to overcome these limitations.
Called aberration-corrected electron microscopes, and invented jointly by ex-ASU physics professor Ondrej Krivanek and a European group, they have ushered in a new era for understanding the properties of materials at the atomic level, using two corrected electron microscopes at the new Southwestern Center for Electron Microscopy at ASU.
“Because of aberration correction, we can see atoms clearly,” said John Spence, professor of physics at ASU, “and because we can see atoms clearly, we can now understand materials’ properties better: why bridges rust, why the blades in aircraft engine turbines fail after so many hours, why semiconductor devices cannot be made any smaller than they are, and what limits the efficiency of the catalysts used in the chemical industry. All of these things can be understood if you can see the atoms.”
In parallel developments over the past 40 years, electron microscopes have been used in biology to image the protein molecules in cells, but these same principles can be now applied to see these molecular machines within organisms at the near-atomic level. These machines perform a myriad of functions in the human body, from making new protein molecules according to instructions from DNA, to healing wounds and digesting food. If researchers can see the molecules within a protein or a virus, then they can understand how it works.
“We have tried over the years several times to get a cryo-EM here; this is the third attempt, and finally all the stars have aligned, since we now have both the machine funded and a new faculty appointment devoted to cryo-EM in the School of Molecular Sciences.” said Spence, who leads to team of ASU researchers who received a three-year, $3.2 million award from the National Science Foundation to make this dream a reality. ASU administration and faculty contributed additional funds, while many faculty across other Southwestern campuses provided supporting letters and a keen interest in using the new machine.
“The microscope has been ordered, it’s being built. It’s a 300kV FEI Krios,” said Tom Sharp, director of the LeRoy Eyring Center for Solid State Science. The 300,000 volts provide the power to peer at the inner workings of biomolecules and materials. “The camera detector system that is a key component of this is the Gatan K2 — that’s the spiffiest camera out there.”
Sense and sensibility
The new cryo-EM doesn’t need to rely on aberration correction to see molecules at near atomic level. The main reason cryo-EM has recently achieved a big increase in resolution, and scientific interest, is due to the invention of a new type of sensor.
Without cryo-EM, biomolecules suffer from severe damage to the sample in an electron microscope, which severely blurred the images in the past. The new camera sensor technology is similar to today’s digital cameras. While previous EM cameras indirectly recorded the electrons, the new cameras for cryo-EM, can directly detect each electron in the beam, and record images at 400 frames per second, so rapidly that damage is minimized. In each exposure, the microscope collects terabytes of data consisting of thousands of images of similar molecules lying in a thin ice layer. Sophisticated software then stitches together these individual molecular images to make one three-dimensional composite image of an undamaged molecule.
“Now if your sample is damaging or moving or changing in any way, you capture it anyway with this rapid collection of images,” said Sharp. “With that now, the computer can put those images together and recreate the structure of your material with a resolution of about three angstroms [The diameter of an atom is around one to two angstroms].”
class="glide image-carousel aligned-carousel slider-start glide--ltr glide--slider glide--swipeable"
id="glide-469657" data-remove-side-background="false"
data-image-auto-size="true" data-has-shadow="true" data-current-index="0">
data-testid="arrows-container">
Clear imaging of biomolecules was once primarily the domain of X-ray crystallography, a technique where researchers crystallize proteins, a process that can often take years just to grow a single crystal. The crystal is exposed to high energy X-rays to produce high-resolution images to determine the three-dimensional protein structure. While cryo-EM of biomolecules has not yet reached the resolution of atoms, it can now provide a detailed view of the molecules frozen in “amorphous ice.” It ideally complements X-ray crystallography methods as it can record images of large biocomplexes — frozen in time and space — at the molecular level that are difficult or impossible to crystallize. “
“What this microscope allows you to do is skip the crystallization. Just disperse the molecules in a frozen slice, and the new cryo-EM instrument can image thousands of molecules automatically, and the computer compares all those images and determines the orientations and so on,” said Sharp.
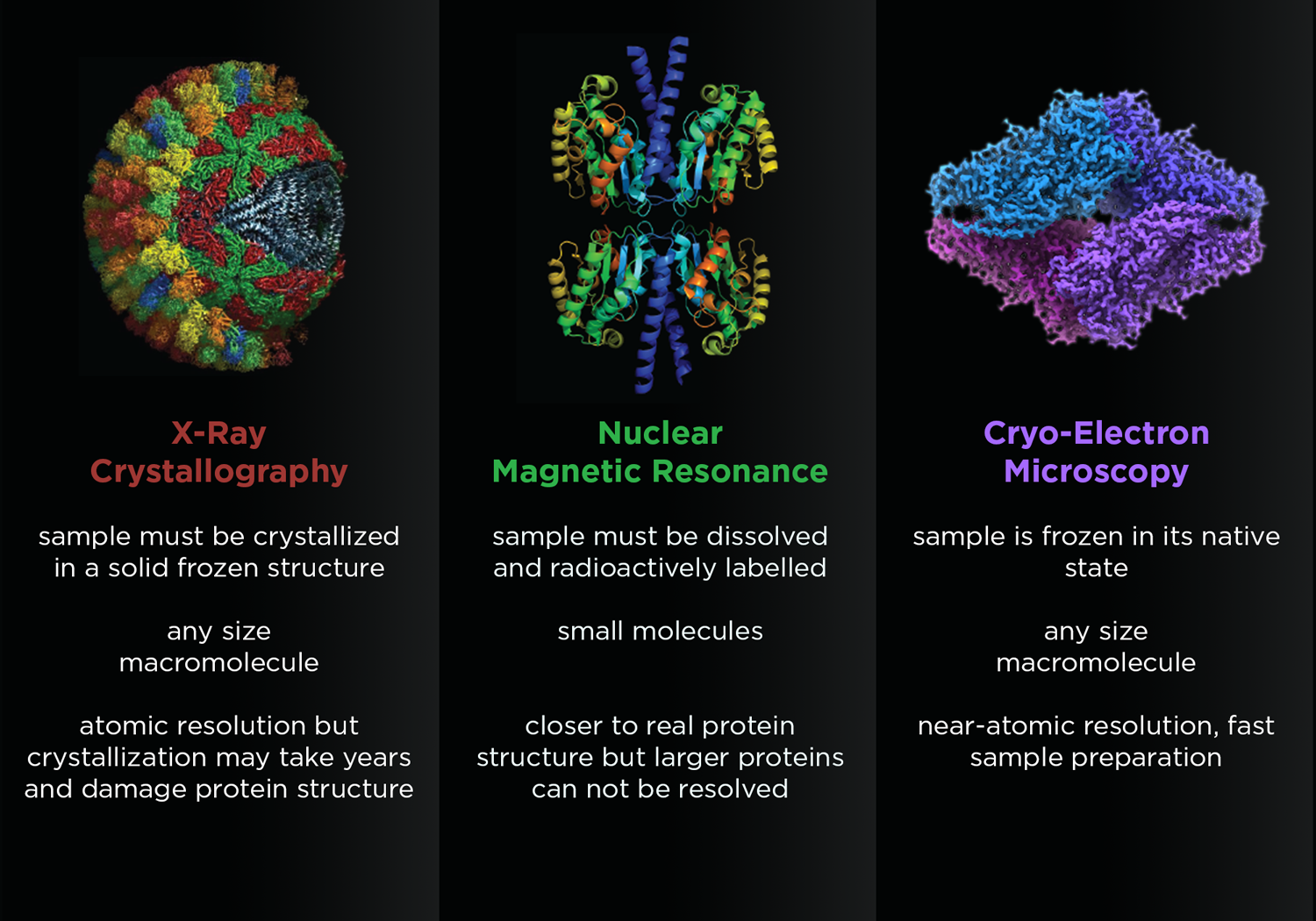
A comparison of three popular methods for looking at biology at work. X-ray crystallography, Nuclear Magnetic Resonance, and now cryo-Electron Microscopy are complementary methods to help structural biologists make better medicines and build new materials. Illustration by Jason Drees
Research powerhouse
This new microscope will be housed in the Southwestern Center for Aberration Corrected Electron Microscopy. What looks like a nondescript building near Hayden library, on the inside holds some of the most powerful imaging tools around.
The building has a special foundation because the center’s suite of instruments is so sensitive — the tiniest of vibrations can ruin an experiment. A sign at the entrance warns not to slam the door — even that, or talking too loudly, is enough to mess up an image being taken by the electron microscopes inside.
Once in place, cryo-EM will serve as a beacon for scientists throughout the Southwest to advance structural biology so they can make better medicines, build new materials, as well as attract new talent to the Valley through faculty hires, students and jobs opportunities for the region.
Written by Ally Carr, assistant science writer, akcarr@asu.edu.
More Science and technology

ASU postdoctoral researcher leads initiative to support graduate student mental health
Olivia Davis had firsthand experience with anxiety and OCD before she entered grad school. Then, during the pandemic and as a result of the growing pressures of the graduate school environment, she…
ASU graduate student researching interplay between family dynamics, ADHD
The symptoms of attention deficit hyperactivity disorder (ADHD) — which include daydreaming, making careless mistakes or taking risks, having a hard time resisting temptation, difficulty getting…

Will this antibiotic work? ASU scientists develop rapid bacterial tests
Bacteria multiply at an astonishing rate, sometimes doubling in number in under four minutes. Imagine a doctor faced with a patient showing severe signs of infection. As they sift through test…